Four AI priorities for biotech and pharma leaders
CAS 2025 Life Sciences Summits recap
In September 2025, CAS hosted Life Sciences Summits in Europe, South Korea, and Japan to explore AI in drug discovery with pharmaceutical, academic, and technology experts. Each summit focused on region-specific priorities, including data governance, AI implementation barriers, and modality-specific challenges. The summits reflected expected trends in drug discovery that are reshaping research strategies.
Across the three CAS Life Sciences Summits, four clear priorities emerged from pharmaceutical companies, biotech organizations, CROs, and academic institutions:
Key themes from leaders:
- Data preparedness over data collection: Organizations have sufficient data volume but struggle with curation, contextualization, and alignment to specific discovery questions.
- Organizational readiness as the bottleneck: Technical capabilities exist, but change management, incentive structures, and cross-disciplinary collaboration determine actual implementation.
- The human-AI partnership model: Rather than replacement, the focus is on repositioning human expertise alongside computational tools.
- Governance frameworks as critical infrastructure: As AI integrates deeper into workflows, accountability and regulatory alignment become essential.
These priorities emerged through in-depth discussions at each summit, revealing how they manifest across different pharmaceutical markets and research contexts.
EMEA: Data and AI to accelerate drug discovery
CAS and AstraZeneca co-hosted the EMEA summit at the Royal Society in London. Sessions covered AI-powered ideation, predictive analytics, automation, and agentic systems.

1. The importance of data readiness debate
Industry participants from pharmaceutical companies, biotech firms, and academic institutions converged on a critical insight: the challenge is not data volume but data readiness. Early sessions focused on what makes datasets effective for AI applications, and participants from pharmaceutical companies and academic institutions described their experiences with implementation. Several noted that poorly curated datasets produced unreliable predictions regardless of model sophistication.
One participant echoed the importance of preparation rather than volume. The data must be curated, contextualized, and aligned with specific discovery questions to produce more relevant, actionable results. Several attendees described similar challenges in their own organizations, reflecting a broader industry realization that many expensive attempts to implement AI stumbled on foundational data issues rather than algorithmic limitations.
The discussions expanded to include approaches beyond traditional published literature. Participants described internal approaches to documenting structured negative results, which record failed experiments that are rarely published but contain valuable information. Others discussed synthetic data generation and federated learning models. Federated learning drew interest because it allows multi-institutional collaboration without sharing proprietary data, though speakers acknowledged that intellectual property concerns and institutional policies remain as barriers to widespread adoption.
2. Cross-discipline connections to drive organizational readiness
Multiple sessions addressed how organizations can connect key elements of collaborative drug discovery including chemistry, biology, computational science, and experimental validation. Some presented collaborative platforms designed to connect teams that traditionally work separately.
The group also discussed cross-company partnerships that pool resources and expertise. While these collaborations can accelerate progress, competitive pressures and concerns over data ownership limit broader adoption.
Industry insight: How to break down cross-department silos. Cross-disciplinary integration emerged as a technical and cultural challenge. Leaders from pharmaceutical and biotech companies noted that connecting chemistry-focused and biology-focused teams requires more than shared data standards. It demands unified governance structures and new incentive models that reward collaboration over individual contributions.
3. Human expertise is necessary for AI adoption
Sessions on large language models and laboratory automation examined how these technologies fit into existing workflows. Presenters demonstrated how large language models can expand chemical design space and generate hypotheses. However, the effectiveness of these LLMs depends on rigorous benchmarking and expert validation. The pharmaceutical industry has been tackling these drug discovery challenges in various ways, with mixed results that underscore the continued importance of human expertise.
Laboratory automation drew attention by reducing experimental variability and shortening cycle times. One speaker described code-driven workflows that enable reproducible experiments across different facilities. However, attendees emphasized that these technologies support rather than replace scientific judgment. Researchers still interpret results, validate predictions, and make strategic decisions about which directions to pursue.
This repositioning of human expertise represents a fundamental shift in how pharmaceutical R&D leaders view AI adoption. Rather than asking "What can AI replace?", the more productive framing became "How does AI expand what researchers can explore?" Several participants from Contract Research Organizations (CROs) and pharmaceutical companies described successful implementations where AI accelerated hypothesis generation while domain experts maintained control over validation, interpretation, and strategic decision-making.
4. How organizations are governing AI tools
A recurring theme was the need for transparent methodologies and clear accountability as AI tools become further integrated. Presenters shared frameworks that track model provenance, documenting decision logic, and validating predictions against experimental outcomes.
One representative outlined their organization's approach to ensuring auditability and managing risk while maintaining scientific rigor. Others raised questions about accountability when AI-generated hypotheses lead to failed experiments or when automated systems produce unexpected results.
“It is encouraging to see leaders across disciplines come together to discuss how artificial intelligence can accelerate scientific breakthroughs, not replace the scientists who drive them,” said Manuel Guzman, president of CAS. “These tools are designed to support researchers by helping them ask better questions, test ideas more efficiently, and make informed decisions.”
Technical capabilities aren’t enough to influence AI adoption
Taken together, these four priorities provided a clear insight for the leaders in attendance: technical capabilities alone don't drive adoption. Participants from pharmaceutical companies and biotech firms emphasized that organizational readiness, incentive structures, and change management processes determine whether teams use AI tools.
The "small wins" strategy. Several pharmaceutical leaders advocated for demonstrating value through targeted successes, such as a validated prediction that accelerates a project timeline, a reproducible workflow that eliminates bottlenecks, or a pilot project that proves the concept. This approach builds organizational buy-in more effectively than enterprise-wide transformations while identifying which AI applications deliver value in drug discovery.
Regional insights
The CAS Life Sciences Summit in South Korea examined how global pharmaceutical organizations apply AI in drug discovery. A featured presenter shared case studies on AI integration in research and development, describing successes and ongoing challenges in real-world settings. Pharmaceutical and biotech leaders attending the summit identified a critical gap: the distance between AI capabilities demonstrated in research settings and practical application in regulated pharmaceutical R&D environments.
The group explored how validation requirements, data quality standards, and risk management considerations influence which tools can be deployed in pharmaceutical R&D. One participant asked how organizations balance the speed AI offers with the rigor required by regulatory agencies.

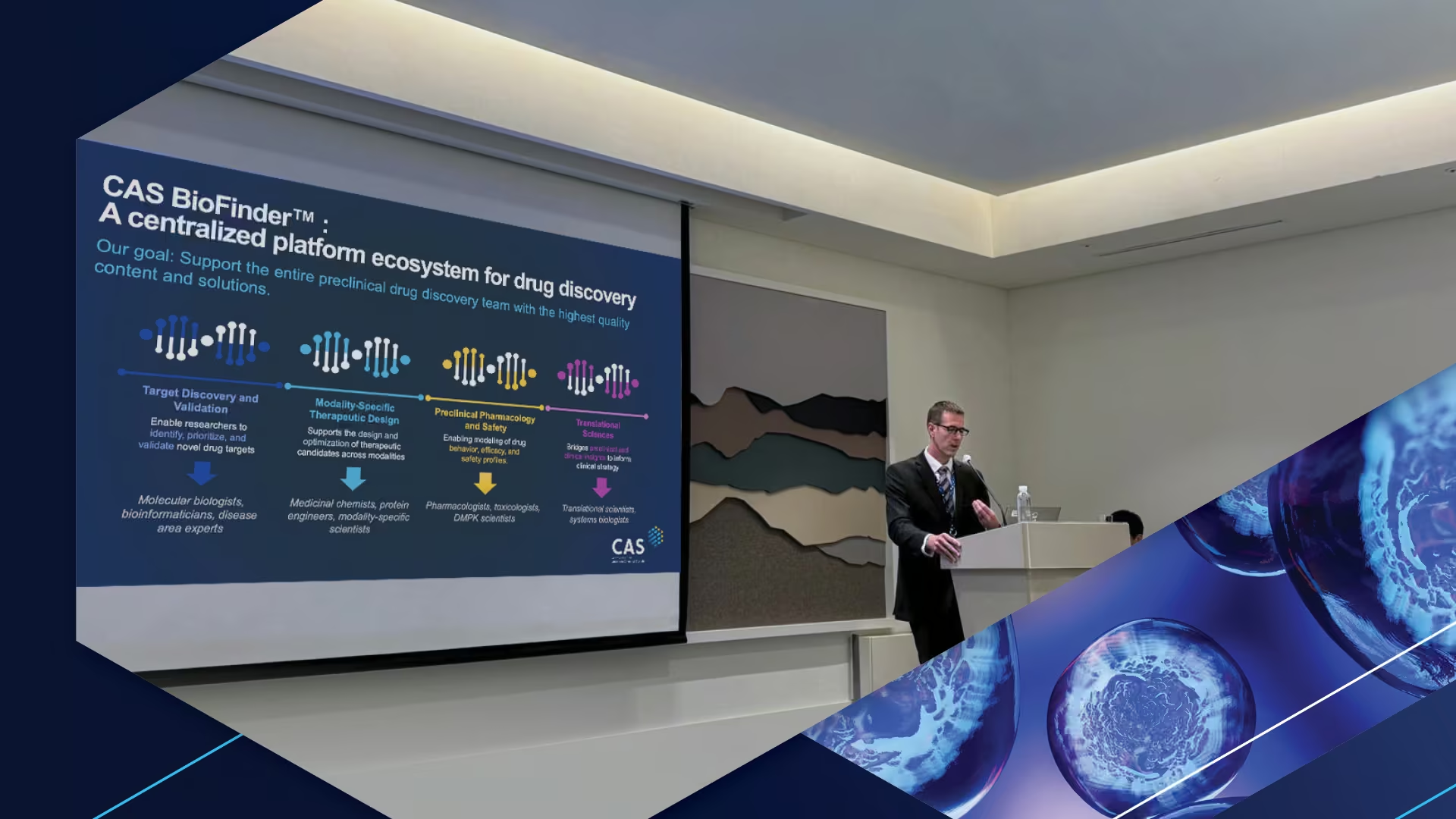
Questions about CAS BioFinder® revealed interest in platforms that connect biological sequence data with chemical information. Several attendees noted that as drug discovery targets become more complex, researchers require tools that integrate diverse data types. This technical challenge mirrors broader organizational discussions about connecting chemistry-focused and biology-focused teams.
As drug discovery focuses more on targets previously considered difficult to modulate, such as protein-protein interactions and challenging binding sites, researchers are exploring approaches, including PROTAC-based protein degradation and small molecule PPI inhibitors.
A notable exchange addressed the role of human expertise in AI–augmented workflows. One participant asked why CAS continues to use human indexers despite the summit’s focus on automation. The discussion revealed that while AI can process large datasets and identify patterns, domain expertise remains essential to curate, validate, and interpret that information.
The conversation concluded that AI expands the scope of what researchers can explore and accelerates hypothesis testing. However, expert judgment regarding biological plausibility, chemical feasibility, and strategic priorities cannot be automated.

The CAS Life Sciences Summit in Japan addressed a question on the minds of many pharmaceutical leaders: Why does AI show promise for small-molecule discovery but struggle with emerging therapeutic modalities such as RNA therapeutics, antibody therapies, peptide drugs, cell therapies, and gene therapies? Guests from pharmaceutical companies, biotech firms, and academic institutions discussed the varying levels of AI readiness across different drug types, which depend heavily on the availability of training data and mechanistic understanding.
The discussion centered around broader trends in drug discovery, examining how AI readiness varies across therapeutic modalities. For small-molecule drug discovery, attendees noted that deep learning models trained on extensive datasets (including target proteins, binding data, and structure-activity relationships for example) can generate hypotheses and predict molecular properties more reliably. These models benefit from decades of accumulated experimental data and well-characterized chemical and biological principles.
Novel modalities present different challenges, presenters explained. RNA therapeutics, antibody therapies, peptide drugs, cell therapies, and gene therapies each face distinct data and mechanistic hurdles. RNA therapeutics, for instance, have limited training data compared to small molecules, and the biological mechanisms governing RNA stability, delivery, and efficacy are less understood. One attendee argued that improving AI accuracy for these modalities requires three parallel efforts: systematically generating high-quality experimental data, developing models that learn from limited examples, and incorporating mechanistic knowledge to constrain predictions within biologically plausible boundaries. Another noted the value of the discussion, describing it as "a rare opportunity to exchange frank opinions across company boundaries in a closed setting.”
Predictive toxicology emerged as a particular concern, as predicting adverse effects from preclinical data remains difficult despite advances in modeling capabilities. Participants called for AI models that can predict efficacy and safety earlier in the discovery process, capabilities that are already emerging in current systems. Looking further ahead, some speakers envision a future where tools could assess the probability of clinical trial success and market potential to support portfolio strategy decisions, though such comprehensive predictive capabilities remain aspirational.
The group identified specific technical gaps, including limited training data for novel modalities, an incomplete mechanistic understanding, and insufficient toxicity prediction capabilities. These gaps point to areas where focused research investment and systematic data generation could accelerate progress.
Building future-ready integrated AI systems
Across all regions, participants agreed that progress requires work on multiple fronts: improving data quality and standardization, enabling collaboration across disciplines and organizations, establishing governance frameworks that build trust, and addressing cultural barriers within institutions. Small demonstrations of value may matter as much as technical breakthroughs in driving broader adoption.
The summits themselves demonstrated the power of cross-organizational collaboration. The events enabled discussions that rarely happen within individual organizations by creating spaces where scientists from competing pharmaceutical companies, biotech firms, CROs, and academic institutions could exchange frank insights. Participants valued the opportunity to focus on advancing scientific understanding rather than competitive positioning, revealing shared challenges and collaborative approaches that might accelerate progress across the entire industry.
As the field moves beyond proof-of-concept demonstrations toward practical implementation, success will depend on leaders who can address critical organizational, cultural, and governance challenges that determine whether AI tools deliver value in real-world pharmaceutical R&D.
For more insights on emerging strategies and technologies shaping the future of drug discovery, explore our 2025 Drug Discovery Trends Webinar.