Small interfering RNA (siRNA) therapeutics are a drug class that uses the RNA interference (RNAi) pathway to silence specific genes. This process was first reported in Caenorhabditis elegans in 1998, and it led to the development of siRNA molecules that target messenger RNA (mRNA) to suppress the production of proteins implicated in various diseases.
In 2006, the scientists who discovered siRNA-mediated gene silencing received the Nobel Prize in Physiology or Medicine. The first approved siRNA therapeutic, patisiran, received regulatory clearance in 2018 for the treatment of hereditary transthyretin-mediated amyloidosis. Since then, additional siRNA drugs have been approved: givosiran for acute hepatic porphyria, lumasiran for primary hyperoxaluria type 1, and inclisiran for hypercholesterolemia management. More recent approvals include vutrisiran and nedosiran, which further extend the application of siRNA therapeutics into metabolic, cardiovascular, and genetic disorders.
These advances established siRNA as a precise therapeutic approach across a range of clinical areas. Conditions from cancer to Alzheimer’s to HIV could be targets of siRNA therapeutics, and according to Pharmaproject, there are over 260 siRNA drug candidates in preclinical or clinical development.
This is promising, but to date there have been significant challenges to consistent, reliable drug development with these therapeutics. Unmodified siRNAs — those administered without chemical modifications or specialized delivery systems — are highly susceptible to rapid degradation by ubiquitous ribonucleases in biological fluids, which drastically reduces their effective half-life and impairs their ability to reach target cells. Furthermore, their inherent negative charge and hydrophilic nature hinder efficient cellular uptake, preventing them from crossing cell membranes and achieving adequate intracellular concentrations. Without chemical modifications, there is also an increased risk of eliciting innate immune responses, potentially triggering off-target inflammatory pathways and compromising overall safety.
Researchers are finding new breakthroughs that may overcome these persistent hurdles. Chemical modifications and advanced drug delivery systems are showing progress, and as a result, siRNA therapeutics are getting closer to enhancing precision medicine across numerous diseases.
How siRNA therapeutics work
siRNA mediates gene silencing via a conserved and regulated RNAi pathway, which facilitates post-transcriptional downregulation of specific gene transcripts (see Figure 1).
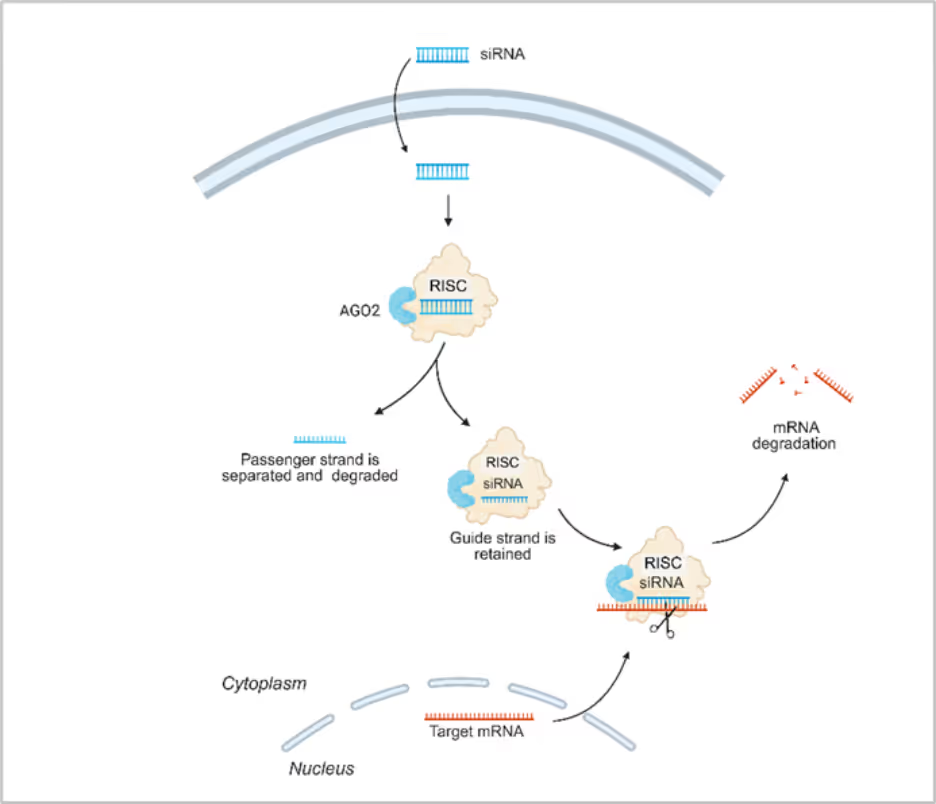
Exogenously administered siRNA duplexes are typically 21–23 nucleotides in length with 3′-dinucleotide overhangs. These are introduced into the cytosol, where they are recognized by the multiprotein RNA-induced silencing complex (RISC). Central to RISC activation is the Argonaute 2 (AGO2) endonuclease, which selectively retains the guide strand following ATP-independent unwinding of the duplex and concomitant ejection of the passenger strand.
The mature, single-stranded siRNA-RISC complex subsequently engages complementary target mRNA transcripts via Watson-Crick base pairing, predominantly within the seed region (nucleotides 2–8). During the next steps of siRNA processing, AGO2 catalyzes site-specific endonucleolytic cleavage of the mRNA between nucleotides corresponding to positions 10 and 11 from the 5′ end of the guide strand. This cleavage disrupts mRNA stability, prompting its degradation by cytoplasmic exonucleases and effectively abrogating translation.
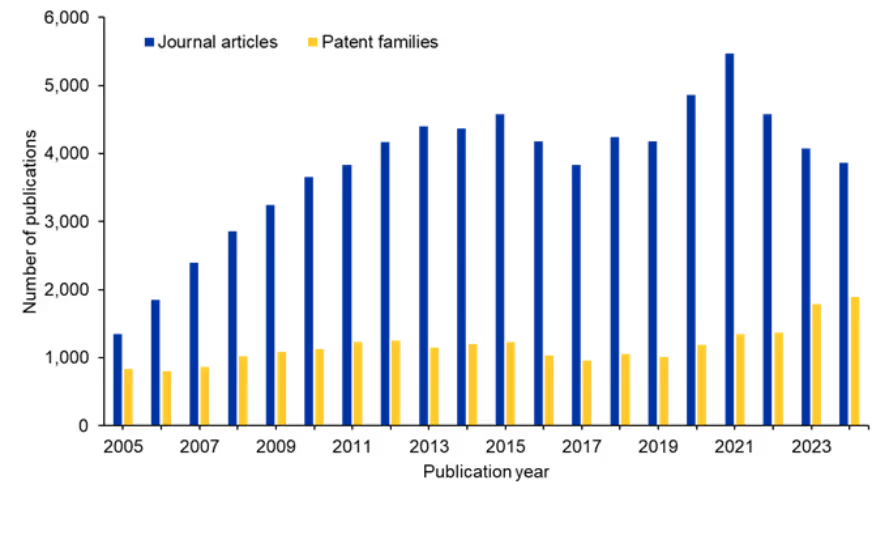
We examined the CAS Content CollectionTM, the largest human-curated repository of scientific information, to better understand the research landscape of siRNA. There is a slightly oscillating trend related to journal publications over the past ten years. However, patent publications continue to increase, suggesting sustained commercial interest in this area (see Figure 2).
As noted, siRNA therapeutics have rapidly evolved into a promising modality across multiple disease areas by precisely silencing genes that drive pathology. According to our analysis of the CAS Content Collection, cancer is the most prominent therapeutic area researched (71%), followed by infectious diseases (8%), neurological conditions (6%), cardiovascular disorders (5%), and diabetes (5%) (see Figure 3).

However, without chemical modifications or advanced drug delivery systems, these therapeutics cannot fulfill their potential to treat diseases effectively. Researchers are making progress in these two areas that could ensure siRNAs can be delivered and maintained at therapeutic levels.
For example, chemical modifications such as the incorporation of 2′-O-methyl, 2′-fluoro groups and phosphorothioate linkages enhance nuclease resistance, improve binding affinity, and reduce immunogenicity, thereby stabilizing siRNAs in circulation. At the same time, advanced delivery systems like lipid nanoparticles (LNPs), polymer-based carriers, and targeted conjugates are critical in overcoming the physical barriers to siRNA uptake by facilitating endosomal escape and enabling tissue-specific distribution. Let’s explore these innovations in more detail:
Chemical modifications of siRNA therapeutics
Chemical modifications address several limitations of unmodified siRNAs, including susceptibility to nucleases, rapid renal clearance, and potential immunogenicity, while also fine-tuning properties such as binding affinity and target specificity. These modifications are generally categorized based on the structural component of the siRNA molecule they affect: the sugar (ribose), phosphate backbone, nucleobases, terminal groups, and conjugation to additional moieties.
The selection and combination of chemical modifications are dictated by several factors: preservation of RNAi activity, stability enhancement, reduction of immunogenicity, pharmacokinetic optimization, and cost-effectiveness. While some modifications offer substantial benefits, they must be balanced against the increased complexity in designing and cost of synthesis for large-scale production.
Sugar (ribose) modifications
Modifications at the 2′-position of the ribose are among the most frequently employed.
- 2′-O-methyl (2′-OMe) groups improve nuclease resistance and reduce immune stimulation.
- 2′-O-methoxyethyl (2′-MOE) modifications further enhance stability and lower toxicity.
- 2′-fluoro (2′-F) substitutions maintain high activity while significantly improving resistance to enzymatic degradation.
- 2′-O-allyl modifications are used to adjust pharmacokinetic properties, offering benefits in systemic circulation.
Conformational modifications such as Locked Nucleic Acid (LNA), with its constrained bicyclic structure, also confer high binding affinity, whereas Unlocked Nucleic Acid (UNA) introduces flexibility into the backbone, potentially reducing off-target interactions. The 2′-4′ bridged nucleic acids are also employed to enhance target specificity through improved conformational rigidity.
Phosphate backbone modifications
The phosphate backbone of siRNAs is critical for molecular stability and cellular uptake.
- Phosphorothioate (PS) modifications involve replacing a non-bridging oxygen with sulfur, significantly increasing resistance to nuclease activity.
- Phosphorodithioate modifications, where both non-bridging oxygens are replaced by sulfur, offer an even greater level of protection.
- Boranophosphate modifications incorporate a borane moiety, altering the backbone’s properties, while methylphosphonate modifications render the backbone neutral, reducing immunogenicity without compromising cellular delivery.
Base modifications
Nucleobase modifications are designed to modulate recognition by immune sensors and alter binding affinities without disrupting Watson-Crick base pairing.
- 5-methylcytosine incorporation can lower immune activation.
- Pseudouridine enhances overall RNA stability while mitigating immune responses.
- 2-thiouridine is used to boost directional binding, and Inosine enables wobble base pairing, which can help reduce unintended off-target effects.
Terminal and conjugate modifications
Terminal modifications further enhance the pharmacological profile of siRNAs. At the 5′-end, modifications such as phosphate removal or temporary silyl protection are used to reduce immune recognition during delivery. At the 3′-end, conjugation of cholesterol or the addition of amino groups facilitate improved cell membrane interaction and uptake. Targeting ligands, including GalNAc for hepatocyte recognition, folate for cancer cell targeting, and aptamers or antibodies for tissue-specific delivery, further refine the biodistribution of siRNAs.
Stabilization and pattern-based modifications
Additional strategies involve tailoring the structure through overhang and internal modifications. Overhang modifications, such as incorporating dTdT nucleotides or chemically modified overhangs, increase resistance to nucleases. Pattern-based modifications include asymmetric and position-specific modifications. For example, heavy modification on the passenger strand versus minimal on the guide strand can bias the RNAi machinery towards the desired functional strand, and targeted alterations at positions critical for RISC loading and activity (e.g., terminal, central, or seed regions) can further refine specificity and activity.
Advanced modifications
Beyond the traditional modifications, advanced analogs such as glycol nucleic acid (GNA), peptide nucleic acid (PNA), morpholino modifications, and tricyclo-DNA represent innovative approaches to altering the backbone structure entirely. These modifications provide alternative methods to enhance stability, specificity, and overall pharmacological behavior, sometimes introducing entirely new properties not attainable with classical RNA modifications.
Delivery systems for siRNA therapeutics
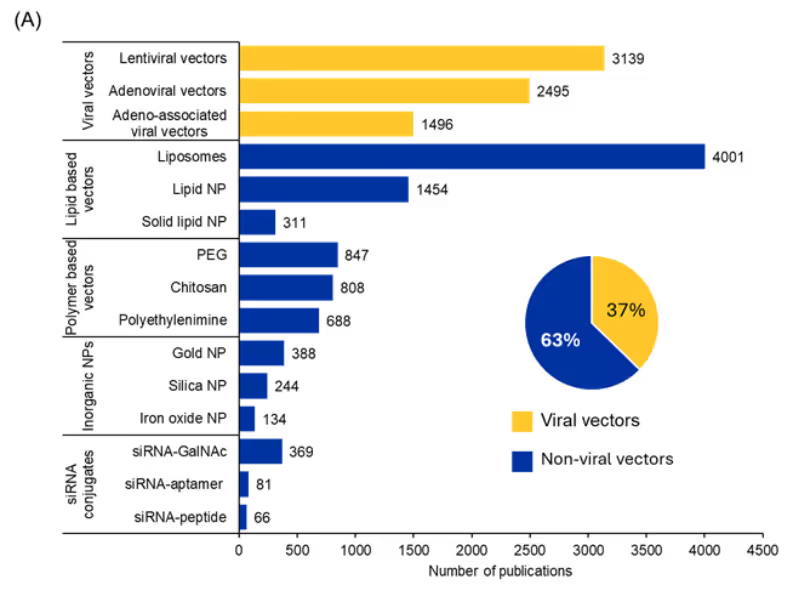
There have been significant advances in siRNA research to address longstanding challenges such as poor stability, low cellular uptake, toxicity, and off-target effects. In our analysis of the CAS Content Collection, we looked for commonly used delivery systems for siRNA administration and found that most publications (63%) mention non-viral delivery systems while 37% mention viral vector-based delivery systems (see Figure 4A).
We further analyzed the trend in the number of publications over the past few years for each of these systems (see Figure 4B). Lipid based vectors show the most prominent increase in terms of publications over the past few years, while siRNA-conjugates also show a subtle increase. Viral vectors and polymer-based vectors have a similar trend, whereas inorganic nanoparticles seem to have plateaued in the last few years.
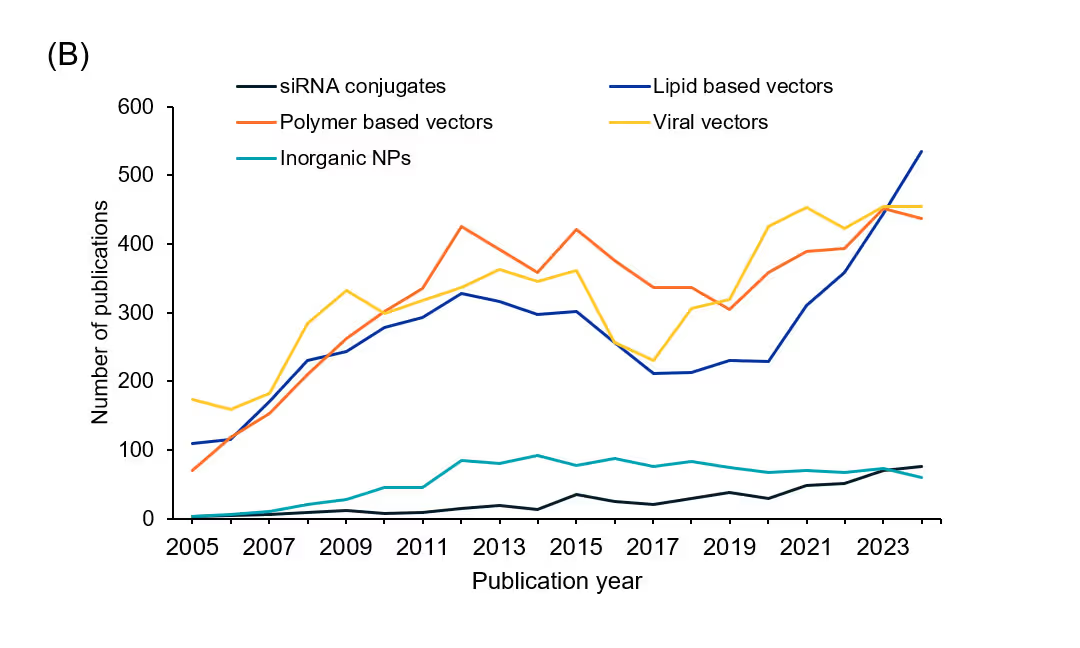
Viral vectors yield high transfection efficiencies due to their natural cellular entry mechanisms and, in some cases, allow for tissue-specific targeting through engineered capsid modifications. For instance, AAV vectors have been explored in clinical trials for neurological and ocular disorders owing to their low immunogenicity and defined tissue tropism, while lentiviral systems continue to be instrumental in preclinical gene silencing studies targeting oncogenes.
Lipid-based vectors remain the most clinically advanced platform. LNPs, which combine ionizable lipids, helper lipids, PEGylated lipids, and cholesterol, are now standard in siRNA delivery, as exemplified by the FDA-approved therapy patisiran for hereditary transthyretin-mediated amyloidosis. Liposomes, in their cationic and neutral forms with added pH-sensitive lipids for better endosomal escape, are also under clinical and preclinical evaluation for numerous indications, including cancer and genetic disorders. Solid lipid nanoparticles (SLNs) and lipoplexes further contribute to the diversity of lipid-based platforms by offering controlled release profiles and scalable production processes.
Polymer-based vectors extend these delivery options by leveraging the electrostatic complexation of siRNA with cationic polymers, thereby enhancing cellular uptake and facilitating endosomal escape. Materials such as polyethylenimine (PEI), poly-L-lysine (PLL), chitosan, and PAMAM dendrimers have been optimized for use in inflammatory, neurological, and oncological diseases. Researchers have also engineered synthetic polymers like poly(β-amino esters) and natural polymers, including hyaluronic acid for targeted delivery, where modifications enable receptor-mediated uptake.
Inorganic nanoparticles have also garnered attention as siRNA carriers. Gold nanoparticles (AuNPs) can be functionalized with cationic polymers to ensure secure siRNA binding and have been evaluated in cancer models for their photothermal release mechanisms. Mesoporous silica nanoparticles, with their tunable pore sizes and surface amino functionalization, have been designed for controlled siRNA release in the tumor microenvironment. Magnetic nanoparticles, composed of iron oxides, offer the dual benefits of directed delivery via external magnetic fields and real-time imaging through MRI, which supports their use in diagnostics and therapeutics.
Direct chemical conjugation strategies provide another efficient route for siRNA delivery. By linking siRNA covalently to targeting ligands such as GalNAc clusters, researchers have achieved hepatocyte-specific delivery via the asialoglycoprotein receptor, which has proven critical for liver diseases. Other conjugated moieties including cholesterol, fatty acids, bile acids, and cell-penetrating peptides enhance cellular uptake and improve pharmacokinetics, thereby broadening the potential therapeutic applications to include cancers and metabolic disorders. It is interesting to note that out of the six FDA approved siRNA therapeutics, five have GalNac modification in them.
Other advanced delivery systems are emerging that capitalize on natural and bioresponsive materials. Exosomes, the cell-derived vesicles known for their innate communication roles and ability to cross biological barriers like the blood-brain barrier, are being refined as carriers for siRNA in CNS disorders. In parallel, bioresponsive materials engineered to release siRNA in response to specific triggers such as pH changes, enzyme activity, redox conditions, or even light exposure are undergoing preclinical evaluation in solid tumor models and pancreatic cancer. Additionally, RNAi microsponges offer a novel approach to RNAi therapy by encapsulating siRNA within a porous, sponge-like structure, potentially improving delivery efficiency and stability compared to traditional siRNA delivery methods.
Collectively, these diverse delivery platforms not only improve the stability, biodistribution, and cellular uptake of siRNAs but also expand the range of clinical applications from liver and genetic diseases to oncology and CNS disorders. The integration of advanced materials science with molecular targeting strategies is paving the way for the next generation of siRNA therapeutics, offering more precise, effective, and safer treatment modalities across various disease areas.
siRNA therapeutics in precision medicine today
Developments in chemical modifications and delivery systems of siRNA therapeutics are driving increased research into using these drugs to treat various diseases. As noted in Figure 3, the most-explored targets include cancer and cardiovascular disease, but there are additional areas of interest:
- Cancer: Preclinical studies and early-phase clinical trials have explored targets like KRAS, VEGF, EGFR, HER2, and c-MYC in various cancers. These targets are key to tumor growth, angiogenesis and immune evasion. Early findings suggest that when these drivers are downregulated, there is a notable effect on tumor regression and metastasis. However, the heterogeneous nature of tumors and the complexity of the tumor microenvironment remain significant challenges, necessitating the development of advanced delivery systems such as tailored lipid nanoparticles or receptor-specific conjugates to ensure the siRNA reaches the intended cells effectively and safely. Recent studies have shown their additional potential in cancer treatment in combination with chemotherapy drugs or immune checkpoint inhibitors.
- Cardiovascular disease: siRNA approaches have already reached a major milestone with the approval of drugs like inclisiran, which targets PCSK9 to robustly lower LDL cholesterol levels. Other targets such as ANGPTL3, APOB, and LPA are under investigation, with early clinical data suggesting that precise hepatic targeting can effectively modulate lipid profiles.
- Neurological and neurodegenerative conditions: The use of siRNAs to target proteins like APP, BACE1, tau, and α-synuclein offers promise for diseases such as Alzheimer’s, Parkinson’s, and ALS. Yet, the blood-brain barrier continues to present a challenging obstacle, prompting the exploration of direct delivery methods (e.g., intrathecal injections) or novel nanoparticle carriers to enhance central nervous system uptake.
- Infectious diseases: siRNAs are being assessed for their ability to silence viral genes as seen in candidates targeting HBV transcripts or HIV regulatory proteins and to modulate host factors like ACE2 in the context of SARS-CoV-2. Although these strategies have shown encouraging reductions in viral load in early studies, rapid viral mutation and potential activation of innate immune responses pose ongoing challenges.
- Diabetes: The translation of siRNA therapeutics into clinical phases for diabetes is still emerging. However, several preclinical studies have underscored the potential of silencing targets such as PTP1B, TXNIP, and FOXO1 to enhance insulin signaling and preserve beta-cell function. The inherent complexity of metabolic regulation and the difficulty in achieving tissue-specific delivery of the siRNAs without off-target effects further complicate the journey toward clinical application. Other studies have used siRNA-mediated silencing approaches to study the role of important proteins like GLUT4, and GSK3B in insulin signaling.
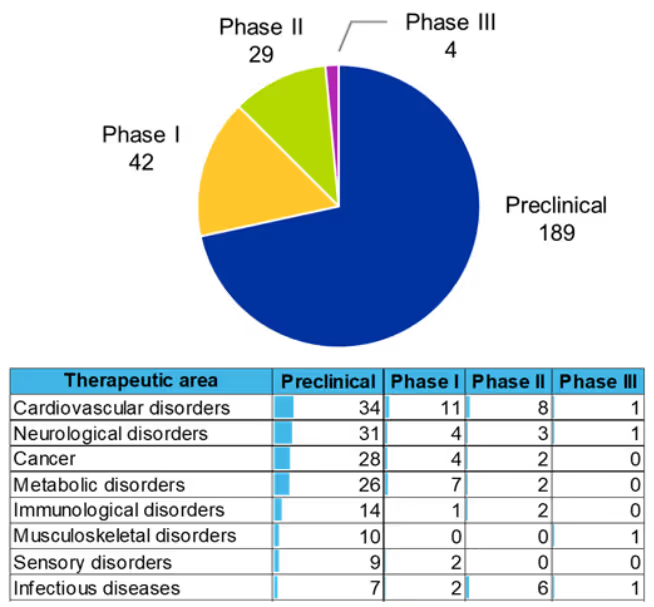
Underscoring the expansion of research into siRNAs is the fact that hundreds of drug candidates are in preclinical and clinical development across these disease categories (see Figure 5). Four drugs are in phase III clinical development, for atherosclerosis (Novo Nordisk/Eli Lilly), Huntington's disease (Ionis Pharmaceuticals/Roche), myotonic muscular dystrophy (Avidity Biosciences), and hepatitis infection (Alnylam Pharmaceuticals/Vir Biotech). Cardiovascular disorders, neurological disorders, and cancers are the top three therapeutic areas for the number of drug candidates in development.
Outlook for siRNA therapeutics
While the liver remains the primary target in siRNA research, current efforts are designing delivery systems aimed at other organs such as the brain, lungs, kidneys, and solid tumors. This involves developing new ligands and nanoparticle formulations that can overcome biological barriers like the blood-brain barrier.
In oncology, siRNA strategies aim to suppress key oncogenes and counteract drug resistance, with promising results emerging from early studies. Advances in lipid-based carriers, polymer vectors, and exosome delivery methods are expected to boost the precision and efficiency of tissue targeting. Enhanced chemical modifications continue to improve siRNA stability and reduce immune responses, resulting in a larger therapeutic window.
These developments could result in wider approval and clinical application of siRNA therapeutics across a range of diseases, offering hope to patients worldwide with important advances in precision medicine.