Lithium batteries are ubiquitous — they power laptops and cell phones, they’re used in battery energy storage systems, and they’re the most common battery technology in electric vehicles (EVs). Demand for lithium will therefore continue to increase. In the first quarter of 2025, EV sales in the U.S. increased by 11.4%, and the IEA estimates that just between 2022 and 2023, battery demand for lithium grew by 30%.
This growth is encouraging since EVs and battery energy storage are important components of reducing greenhouse gas emissions. However, as demand for lithium grows, supply issues will become more acute. Global lithium reserves from land-based resources are estimated to be about 22 million metric tons and high concentrations are available only in a handful of geographic locations, particularly high-grade brines in various South American countries and China, and hard-rock mines in Australia.
{{microelectronics="/ads"}}
Not only could supply become an issue as demand increases, but the process of extracting lithium from these sources also presents challenges. Lithium extraction from brines involves solar evaporation and chemical precipitation, a process that is time-consuming and can alter local ecosystems and water supplies. Hard-rock mining also causes environmental impacts and involves high chemical usage and water consumption.
To confront these challenges, researchers are exploring new ways to extract lithium with the goal of eventually deriving this element from seawater. There are about 200 billion metric tons of lithium in oceans — a nearly inexhaustible supply — but the concentration is extremely low, at just 180 parts per million, and it’s surrounded by extensive amounts of other ions like sodium.
How can we improve extraction to access these ocean-based supplies? By repurposing and refining technology used in desalination, we can improve the extraction of lithium from brines, which we can eventually extend to seawater.
Direct lithium extraction opens new possibilities
Direct lithium extraction (DLE) aims to efficiently capture lithium ions from brines, including brines from oil and gas fields as well as geothermal brines and salt lakes. There are several methods for carrying out this process, most of which are adapted from existing technologies for desalination and wastewater treatment. We analyzed the CAS Content CollectionTM, the largest human-curated collection of scientific information, to better understand how these technologies are changing, and we found that publications related to DLE have grown at a rapid pace in recent years (see Figure 1). The last two years saw precipitous growth, and in just the first two months of 2025, the number of publications matched one-third of the total for 2024.
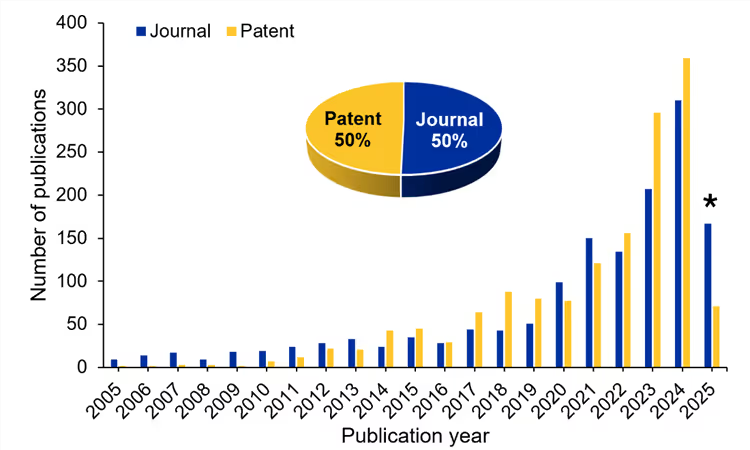
The fact that half of all publications are patents indicates high commercial interest in these technologies. This makes sense given the growth in demand for lithium-powered batteries. DLE also has numerous advantages over current extraction methods — less water and chemical consumption, minimal environmental impact, high recovery rates, faster extraction, and potentially lower costs.
However, each brine contains different levels of lithium, other elements and ions, and total dissolved solids, which means that one method isn’t effective in every situation. Multiple technologies are being investigated in the growing literature around DLE, each with unique advantages and disadvantages.
Types of direct lithium extraction
There are five types of DLE for use with brines and potentially seawater:
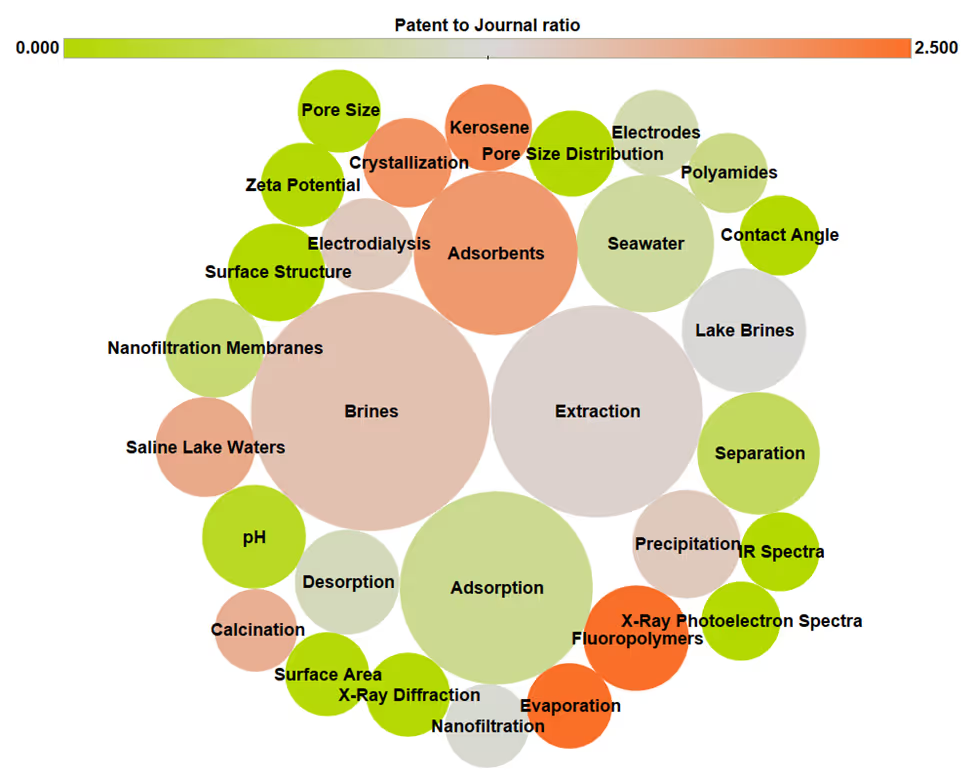
- Adsorption: Adsorption with aluminum-based materials is currently the only commercialized DLE technology. When we analyzed publications in the CAS Content Collection for concepts in DLE-related literature, “adsorption” and “adsorbents” were among the highest-reported concepts (see Figure 2). This demonstrates how widely studied this method is. The process works by adsorbents selectively binding lithium, then using a different solution to de-sorb it. Carbon, zeolites, metal-organic frameworks, and layered double hydroxides are examples of materials used in this process. The advantages of adsorption are its potential for high lithium uptake and low energy consumption. However, as researchers have noted, to reach its highest Technology Readiness Level (TRL), adsorption requires a minimum lithium content of >100 mg/L, high brine temperatures around 50℃, and sufficiently high salinity.
- Ion exchange: This process involves the exchange of lithium ions with different ions carrying the same charge inside of adsorbents. Similar to adsorption, this process has a high lithium uptake capacity. However, acidic solutions are used with intercalation materials, and these materials can become unstable or degraded due to the acids. Intercalation materials include manganese and titanium.
- Solvent extraction: This method relies on the separation of lithium based on the difference in partition coefficient of lithium between organic and aqueous phases. Solvent extraction is effective with complex brines, but it uses kerosene or methyl isobutyl ketone, which increases fire risks. There are also environmental concerns around the use of additives and solvents with this technology.
- Membranes: Semipermeable membranes can be used to selectively filter lithium ions and other salts in a brine. These membranes can be polyamide, NASICON-based materials, or reverse osmosis membranes. The process is relatively simple and environmentally friendly, and it doesn’t require high energy usage. However, this method requires pre-treatment for high-concentration brines, so it’s not efficient for every application.
- Electrochemical: This process is still in the applied research phase, but it’s showing promise. It involves the use of lithium intercalation materials as electrodes for extraction, and it can be applied to lithium-ion battery leachate, which repurposes material that would otherwise be wasted. Researchers have noted its high lithium selectivity, short reaction time, and low energy consumption. One study showed lithium manganese oxide and activated carbon electrodes were reusable with no degradation. Lithium nickel manganese oxide (LNMO) and lithium iron phosphate (LFP) are also potential electrode materials. This process still needs refinement before it’s ready for commercialization, but a Stanford-led study used it in seawater at Half Moon Bay in California and saw positive results.
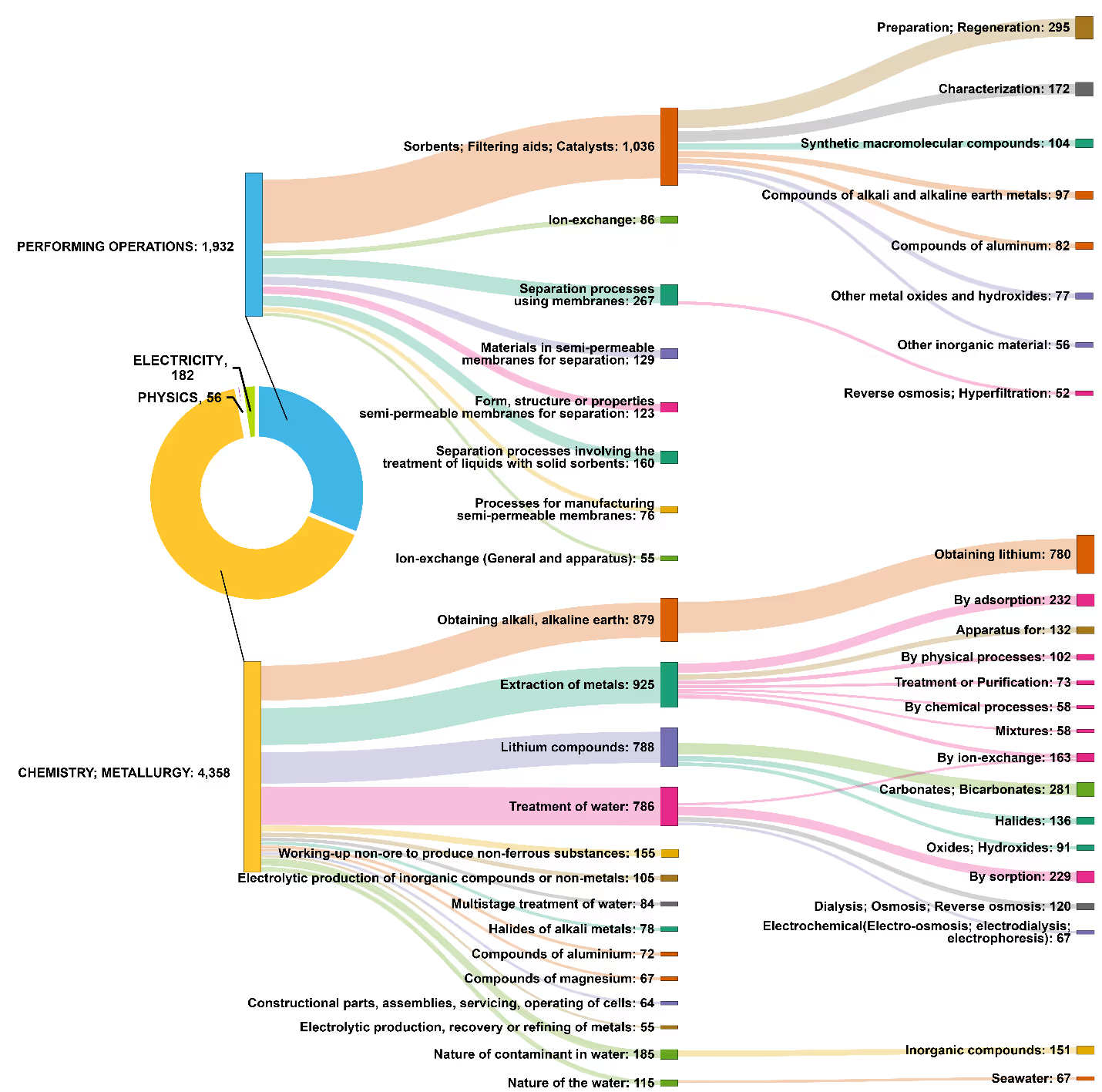
We further analyzed the research landscape by evaluating International Patent Classification (IPC) codes related to DLE. Most patents belong to the IPC sections of “Chemistry; Metallurgy” and “Performing Operations.” The top DLE methods in this analysis corresponded to our concept analysis, namely adsorption as the leading method followed by membrane-based technologies (see Figure 3).
Challenges to extracting lithium from seawater
Before lithium can be efficiently extracted from seawater at a large scale, these methods must be refined with available brines and improved to the point that they can extract large amounts of lithium from the low-concentration source of seawater. There are hurdles to this process, including cost since these extraction methods are currently more expensive than a method like solar evaporation. Furthermore, lithium prices must remain stable for these technologies to be economically feasible.
Scalability is another challenge — not only must these extraction methods be able to be used on a large-scale basis, but ideally one or more methods will be usable with various types of brines so that all available aqueous resources can be accessed. With these types of improvements, it’s possible that we may be able to extend these DLE technologies to seawater and prevent any future lithium shortages.
These are significant challenges, but as lithium batteries become even more common in cars and personal devices, the impetus to find breakthroughs will drive more research and better technologies.







