Spatial transcriptomics marks a significant advancement in biological research, offering a powerful technique to examine the intricate relationship between gene expression and spatial organization within an intact tissue. By revealing the spatial arrangement of cellular components and their transcriptional states, this method allows a deeper understanding of cellular composition and activity in complex biological systems.
Spatial transcriptomics encompasses several methods that share the goal of assigning transcriptomics data to the original location of the RNA molecules within the tissue. This technological advancement provides unprecedented insights into the organization, function, and interactions of cells within complex tissues. Because of its potential to improve numerous biological and medical fields, such as developmental biology, neuroscience, and cancer research, spatial transcriptomics was recognized as “Method of the Year” by Nature Methods in 2020.
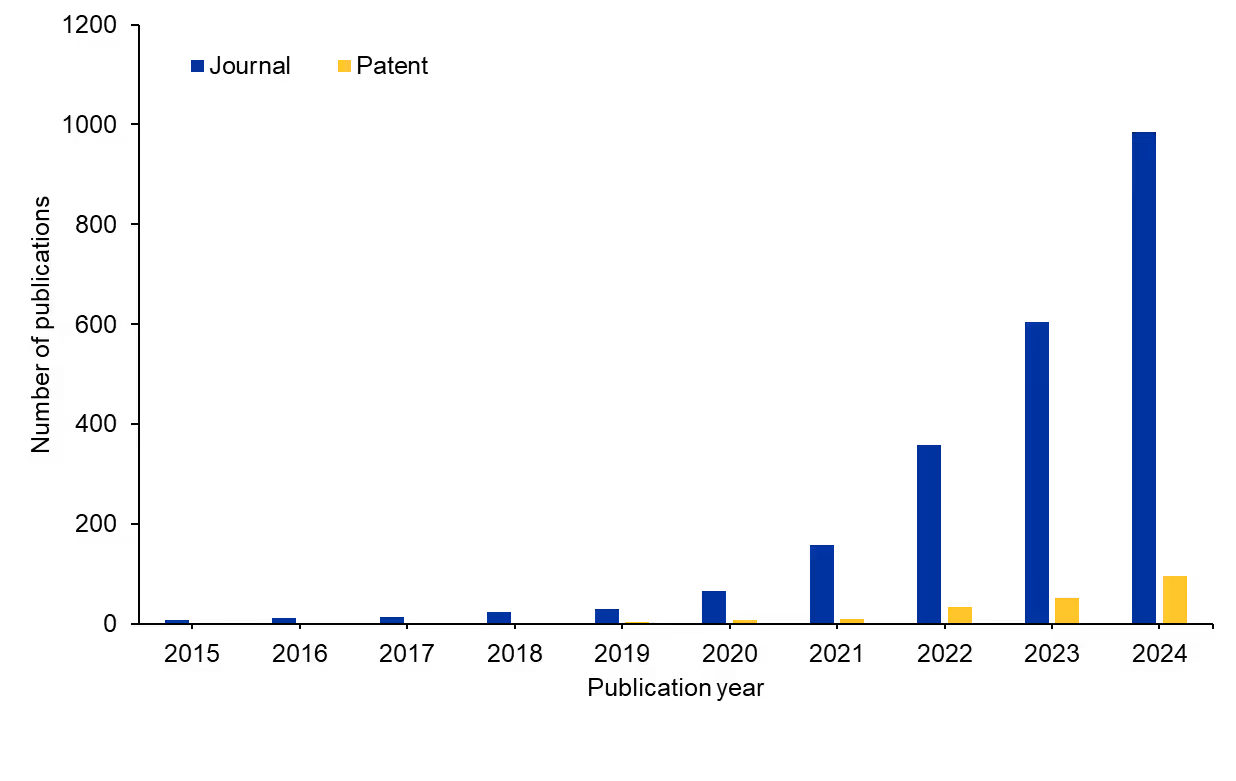
Other transcriptomics approaches exist in biomedicine, but researchers have made exciting strides with spatial transcriptomics recently. We examined the CAS Content CollectionTM, the largest human-curated repository of scientific information, to better understand the research landscape of spatial transcriptomics over the past decade. We saw an enormous increase (around 150-fold) in publications relating to spatial transcriptomics starting 10 years ago (see Figure 1). Journal publications dominate the literature compared to patent publications, suggesting there is still a major opportunity for commercialization.
How is the science of transcriptomics changing, and what does this mean for new treatment approaches for various diseases? Let’s explore how spatial transcriptomics work and where the research is headed.
What is spatial transcriptomics?
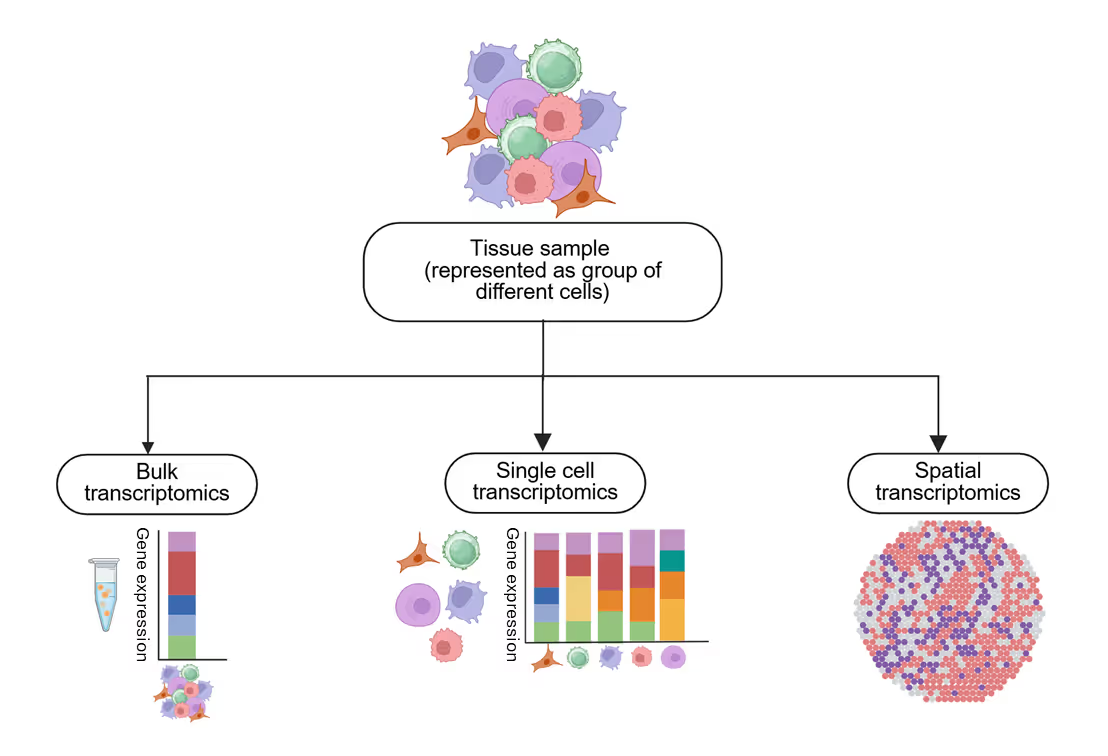
There are currently three main types of transcriptomics: bulk, single-cell, and spatial (see Figure 2). Bulk transcriptomics analyzes gene expression in a mixture of cells, providing a global view but lacking resolution at the single-cell level. Single-cell transcriptomics resolves gene expression in individual cells, enabling insights into cellular heterogeneity and rare cell populations, but it loses the spatial context.
Spatial transcriptomics preserves the location of gene expression within tissues, revealing cellular interactions and tissue architecture, though it must be noted that this technique requires sophisticated computational analysis. Together, these approaches offer complementary views of gene regulation, each suited for different research questions.
One of the earliest spatial transcriptomics techniques was FISH (fluorescent in situ hybridization), which directly visualizes RNA molecules within intact cells and tissues. It works by designing short, fluorescently labeled DNA or RNA probes complementary to the target RNA sequence. These probes hybridize to the target RNA in the sample, and the locations of the fluorescent signals are detected using fluorescence microscopy.
Techniques like single-molecule FISH (smFISH) improved sensitivity, allowing for the detection and quantification of individual RNA transcripts as diffraction-limited spots. Initial applications of FISH could only visualize the spatial distribution of a small number of well-characterized mRNAs, due to spectral overlap of fluorophores. These early techniques were also time-consuming and labor-intensive, especially when analyzing large tissue sections or many targets. Due to these limitations, researchers developed modern methods with higher efficiency.
Technological advances improve resolution and analysis
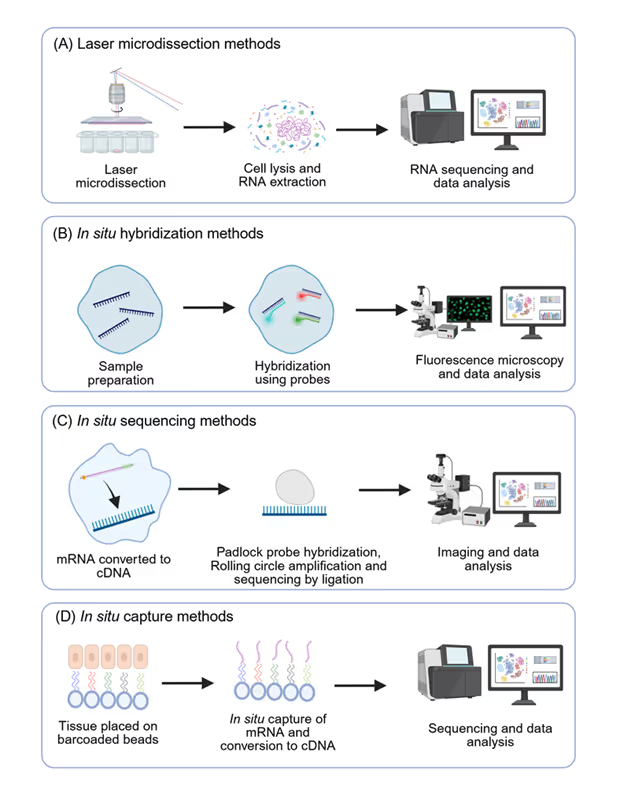
Spatial transcriptomics technologies can be divided into four categories: microdissection methods, in situ hybridization methods (ISH), in situ sequencing methods (ISS), and in situ capture methods (ISC) (see Figure 3). The choice of technology often depends on specific research goals, such as the need for high resolution, broad gene coverage, or targeted analysis. As we saw in the publication numbers in the CAS Content Collection, this field has experienced rapid technological evolution in the past decade. These advances enable researchers to identify critical insights into disease mechanisms and develop potential treatment strategies.
Laser Capture Microdissection methods
Laser capture microdissection (LMD) provides a physical means to isolate specific cell populations or regions of interest directly from tissue sections under microscopic visualization. Once the desired areas are microdissected, the RNA is extracted from these isolated cells or tissue fragments, then analyzed using traditional bulk RNA sequencing (RNA-seq). This approach links gene expression profiles to specific spatial locations defined by the microdissection.
While LMD focuses on spatial isolation rather than in situ analysis, its integration with advanced RNA-seq technologies has significantly enhanced its utility in spatial transcriptomics. Improvements in RNA extraction from small numbers of cells, amplification methods, and sequencing depth have enabled more comprehensive transcriptome analysis of microdissected samples. Newer LMD instruments offer higher precision and automation, making the process more efficient. These can prove crucial for understanding the molecular heterogeneity within tissue samples, identifying specific cell populations that are driving disease, and potentially guiding targeted therapies.
ISH methods
ISH encompasses many techniques that use labeled probes to detect specific nucleic acid sequences (both DNA and RNA) within cells or tissues while preserving their spatial context. Similar to FISH, ISH relies on the hybridization of labeled probes to target sequences. However, ISH can employ various detection methods beyond fluorescence, including enzymatic or radioactive labels, which can be visualized using microscopy or autoradiography.
Traditional ISH using chromogenic detection (e.g., using enzymes that produce a colored precipitate) has been a fundamental technique in developmental biology and histology for decades, allowing visualization of gene expression patterns at cellular resolution. Radioactive ISH offers higher sensitivity for low-abundance transcripts. FISH is comparable to a fluorescence-based subset of ISH.
The evolution of ISH has largely focused on increasing sensitivity, multiplexing capability, and resolution. Recent multiplexed FISH like MERFISH, seqFISH, and other variants enable the simultaneous detection of hundreds to thousands of mRNAs within the same sample by employing combinatorial labeling and sequential rounds of hybridization and imaging.
ISS methods
These methods typically involve converting RNA into complementary DNA (cDNA) within the tissue section, followed by in situ amplification and sequencing using various encoding strategies. The sequence information is then decoded through iterative rounds of hybridization, ligation, or enzymatic reactions, coupled with high-resolution imaging. The resulting data provides the identity and spatial location of numerous RNA transcripts at subcellular resolution.
Examples of ISS technologies include Single-cell TOtal RNA Miniaturized sequencing (STORM-seq), fluorescent in situ sequencing (FISSEQ), and padlock probe-based sequencing methods. These techniques can achieve high multiplexing, allowing the simultaneous detection of thousands of RNA species within individual cells in their native spatial context.
ISC methods
ISC methods involve placing spatially barcoded oligonucleotides on a tissue section to capture the released mRNA from the cells. The spatial information is encoded in the unique barcodes on the capture probes. After mRNA capture and reverse transcription, the resulting cDNA is pooled and sequenced using standard next-generation sequencing. By decoding the spatial barcodes associated with each cDNA molecule, the gene expression profile can be mapped back to its original location on the tissue.
Table 1: Comparison between different ST technologies
The original spatial transcriptomics technology, developed at the Karolinska Institute, is a prime example of an ISC method, which was commercialized later as the 10x Genomics Visium platform. It uses spatially barcoded microarrays to capture mRNA. Subsequent innovations include Slide-seq, which uses DNA-barcoded beads deposited on a slide.
Initial ISC methods had relatively low spatial resolution, limited by the size of the capture spots or beads. Significant progress has been made in increasing the density of spatial barcodes, reducing the capture area, and improving the efficiency of mRNA capture and cDNA synthesis. Techniques like high-definition spatial transcriptomics (HDST) and Slide-seqV2 have dramatically increased the resolution, approaching single-cell levels in some cases.
ISC methods offer a scalable and relatively cost-effective approach for whole-transcriptome spatial profiling, making them attractive for large-scale studies in basic research, drug development, and potentially clinical diagnostics. The ability to analyze the entire transcriptome with spatial context provides a comprehensive view of tissue organization and function.
Spatial transcriptomics improving our understanding of disease biology and drug discovery
Understanding the spatial context of gene expression provides critical insights to unravel disease mechanisms. It also affects drug discovery and development. The four focus areas of spatial transcriptomics today are cancer, neuroscience, developmental biology, and infectious diseases.
Cancer
One of the most significant challenges in cancer research has been understanding and addressing tumor heterogeneity and the complex interplay between tumors and their surrounding microenvironment (TME). TME is a complex and continuously evolving entity which typically includes immune cells, stromal cells, blood vessels, and an extracellular matrix. Thanks to advances in spatial transcriptomics, the vital role of the immune microenvironment is now better understood, revealing the various mechanisms that cancer cells use to bypass the host immune system.
It is now broadly accepted that TME is an active promoter of cancer progression and not just a neutral entity. It directly or indirectly regulates cancer growth, metastasis, resistance, or susceptibility to various treatments. As spatial transcriptomics techniques preserve spatial context, these are excellent tools to study tumor-TME and tumor-immune cells’ interactions and provide novel insights into our understanding of tumor biology.
There have been numerous notable studies recently. For example, in glioblastoma, IL-10-releasing myeloid cells (located in mesenchymal-like tumor regions) were shown to drive T-cell exhaustion, contributing to immunosuppressive TME. In oral squamous cell carcinoma, distinct and conserved tumor core and edge architectures were identified and characterized, which predicted survival and targeted therapy response. Spatial transcriptomics is also widely used in breast cancer research to study tumor heterogeneity and TME-driven drug response.
[Add breaker]: PPI inhibitors show promise in fighting cancer [link]
Neuroscience
The brain’s remarkable functional complexity arises from the precise spatial arrangement and connections between the diverse neuronal and glial cell populations. Spatial transcriptomics has the capability to dissect this organization at the cellular level and provide insights on how neuronal circuits are formed and become functional, how different brain regions are specified, and how disruptions of this finely tuned architecture can lead to the onset and/or progression of various neurological disorders.
Spatial transcriptomics is now used to decipher critical mechanisms and molecular players in amyloid plaque pathology, a hallmark of Alzheimer’s. In mouse models, scientists have identified gene co-expression networks involved in the early phase versus the late phase of the disease (myelin and oligodendrocyte genes [OLIGs] and plaque-induced genes [PIGs], respectively). In another study, associations between regional gene expression profiles and selective regional vulnerability to amyloid-beta and tau pathologies in Alzheimer’s patients were identified.
Spatial transcriptomics has also helped us uncover potential molecular targets that could enhance amyloid beta-targeted immunotherapies. Researchers are studying the neuroimmune mechanisms involved in the elimination of amyloid-beta plaques in patients who received active or passive immunization (upregulation of genes associated with the TREM2-APOE axis). Also, a novel computational framework to integrate “Spatial Transcriptomic data using over-parameterized graph-based Autoencoders with Chromatin Imaging data (STACI)” was used to identify potential new Alzheimer’s biomarkers.
Apart from Alzheimer’s research, spatial transcriptomics has provided novel insights on several risk genes and pathway dysregulations in amyotrophic lateral sclerosis (ALS).
Developmental biology
Several studies have highlighted the power of spatial transcriptomics in developmental biology. By applying the Stereo-seq method to generate the mouse organogenesis spatiotemporal transcriptomic atlas (MOSTA), researchers have gained valuable insights into the molecular basis of spatial cell heterogeneity and cell fate specification in developing tissues such as the dorsal midbrain. In another study, spatial transcriptomics was used to profile spatiotemporal gene expression patterns in human cardiogenesis and map the cell-type distribution and spatial organization in the human embryonic heart.
In another development, an entire transcriptomic brain atlas of the mouse brain was generated with spatial information for 308 cell clusters at single-cell resolution, involving over 4 million cells. This provides a valuable resource for studying development, function, and gene regulation of the mouse brain.
Infectious diseases
Spatial transcriptomics is transforming the study of infectious diseases by providing the ability to analyze the intricate interactions between host and pathogen within the spatial context of infected tissues. This methodology has been successfully employed to study bacterial infections such as Mycobacterium tuberculosis, Pseudomonas aeruginosa, and Echinococcus multilocularis. It is also used in research into viral infections, such as SARS-COV-2 and HIV. Researchers have directly identified certain co-localization patterns of host and pathogen transcripts and discovered host factors that modulate immune response.
Challenges and opportunities in spatial transcriptomics
While spatial transcriptomic approaches offer exceptional insights, they also come with certain challenges. The data generated by most spatial transcriptomics methods is huge, complex, and sometimes noisy because of low RNA capture efficiency, PCR amplification biases, or sequencing errors. This often requires sophisticated algorithms for image processing, spatial mapping, and integration with single-cell RNA sequencing. Tissue fixation, sectioning, and staining can introduce artifacts that impact RNA integrity and spatial resolution. Handling and processing techniques need to be optimized to minimize these issues.
The reagents, sequencing, and computational infrastructure required for spatial transcriptomics can be expensive, limiting the availability of these innovations. Also, these approaches involve complex experimental workflows, requiring researchers with expertise in molecular biology, bioinformatics, and microscopy. Interdisciplinary skills are essential for accurate analysis and interpretation. Furthermore, spatial transcriptomics protocols considerably vary across labs, making it challenging to compare results across studies.
Despite these challenges, the field of spatial transcriptomics continues to evolve rapidly, with ongoing efforts focused on enhancing spatial resolution, increasing sensitivity, and improving throughput. Another possibility is integration with other omics data, known as spatial multiomics, which could provide a more comprehensive understanding of biological and pathological processes by combining genomics, proteomics, epigenomics, and metabolomics — all within a spatial framework.
Ongoing advancements are improving the data analysis methods and reducing noise for better comprehension of ST data. Like many other biomedical fields, AI is set to revolutionize spatial transcriptomics by making data analysis more efficient and insightful. Some recent examples include Path2space, a deep learning model designed to predict spatial gene expression directly from histopathology slides of breast cancer biopsies and predict patients’ response to chemotherapy and trastuzumab, and Spotiphy, a machine learning-based approach to achieve single-cell resolution and whole-genome coverage. In vivo spatial transcriptomics methodologies are still in their early stages, but will soon be achievable thanks to ongoing research efforts.
Looking ahead, the future of spatial transcriptomics is promising. It is poised to drive further groundbreaking discoveries in basic research and have an increasing impact on translational medicine.