Smartphones, laptops, Bluetooth speakers, electric vehicles (EVs) — these technologies are part of our everyday lives, but rarely do we consider the metallic elements that make them function. The central processing units (CPUs) and graphical processing units (GPUs) in our electronic devices are made of silicon, and those chips take care of the calculations. However, there are other equally important circuitry components, such as hard disk drives and flash memory. Without these, we would be unable to keep large amounts of temporary or long-term stored data while browsing or performing demanding tasks. The raw materials of these components are made of what’s known as critical metals.
Critical metals are considered as such for two reasons: their crucial roles in modern industries and their uneven abundance and geographic distribution. These factors make them highly desired but more difficult to obtain in regions and countries that lack significant natural deposits. Rising protectionism and trade barriers are exacerbating supply and demand imbalances. Price volatility is also expected to persist as these imbalances continue.
{{microelectronics="/ads"}}
These factors show why the world needs more efficient and effective processes for extracting critical metals. Current methods are commonly performed with toxic chemicals and harsh reaction conditions, making these steps environmentally unfriendly and inefficient with significant solvent use. Furthermore, a significant degree of critical metals are lost in the purification process due to poor separation technology.
Fortunately, significant scientific research is addressing these issues, and better methods may soon meet market needs and environmental imperatives.
What are critical metals?
When looking at the Periodic Table of Elements (see Figure 1), critical metals include some alkaline earth metals like lithium, some first-row transition metals like cobalt, and some p-block metals like tellurium. They also comprise second- and third-row transition metals encompassing the platinum group metals (PGMs) — platinum, palladium, rhodium, ruthenium, iridium, and osmium. Critical metals also include some of the lanthanides or rare earth metals (REMs), also known as rare earth elements (REEs), including lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, and terbium.

The United States Geological Survey (USGS) monitors a larger set called critical minerals including the elements aluminum, copper, iron, and nickel, but we are not considering those in our analysis due to their relative abundance and established purification processes.
Not only are critical metals highly valuable and unevenly distributed, but they have also replaced lesser-performing metals due to their improved physical properties. For example, some critical metals such as neodymium have incredibly strong permanent magnetic properties, more so than iron, which makes them ideal for high-performing memory devices in smartphones, computers, and cloud computing data servers, thus being indispensable components in the digital age.
It is worth noting the amount of critical metals each country or region has for the elements in question. USGS provides global coverage of these natural resources with regular reports and updates. Table 1 shows a heat map of the USGS data for the reserves in metric tons (MT) per country or region; note that USGS reports the elements in the PGMs and the lanthanides (REEs) as two combined entities, while lithium, cobalt, and tellurium are distinguished. As expected, tellurium has very little abundance, with reserves reported for Canada to be just 0.9K MT, 3.1K in China, 3.8K in the United States and 5.8K in Russia. Contrast this with 57M MT of lithium in Australia, or 44M MT of REE in China and 1.8M MTs in the U.S.
.avif)
Applications of critical metals
Critical metals are used in numerous technologies and devices, but how are they specifically incorporated? There are three main applications:
- Batteries/energy storage: Lithium and cobalt are used in batteries for their charge density and cathode stability for long-lasting life cycles. No other elements have been found that provide the same performance. For example, low-cost and widely used lead and zinc-based batteries have been commercially available for many years, but they have environmental concerns and poorer charge retention and battery life span.
- Magnets: Data centers require magnetic materials to maintain performance at elevated temperatures, smartphone cameras need ultra-precise magnetic actuators for image stabilization, and EV motors demand magnets that retain strength under stress. Neodymium is one of the world’s strongest permanent magnetic metals commercially available; dysprosium maintains magnetic strength at high temperatures; and terbium can be used to enhance magnetic properties of other components. Critical metals such as praseodymium offer a relatively lower-cost alternative solution to neodymium in some cases. The typical memory components they are involved in include hard disk drives for high-density data storage, magnetic RAM for non-volatile memory, and miniature magnets for speakers and headphones.
- Catalysis: Out of the critical metals used in catalysis, ruthenium is vital for ammonia production and iridium for green hydrogen production (water electrolysis). Catalytic converters are widely used to reduce various harmful gases from automotive exhausts and include platinum to convert carbon monoxide, rhodium to reduce nitrous oxides, and palladium which can convert unburnt hydrocarbons.
Improving extraction, separation, and purification of critical metals
The industries that use critical metals already face significant challenges due to their lack of abundance. In addition, once devices have reached the end of their life cycle, they’re typically thrown into landfills. Recent efforts have focused on recycling the devices to extract those critical metals and reuse them in new devices. While this has proved successful to some degree and should be a priority, there are other ways to improve extraction rates for critical metals at the beginning of their life cycles such as mining, separation, and purification.
There are currently two typical processes for the extraction of critical metals from other elements: hydrometallurgy and pyrometallurgy, each being an umbrella for specific processes. Hydrometallurgy includes acid leaching, alkaline leaching, solvent extraction, and ion exchange processes. Pyrometallurgy includes high-temperature smelting and roasting, calcination to remove volatile impurities, and a reduction process using carbon or hydrogen.
Each of these methods presents environmental risks, such as high energy usage, usually achieved by burning fossil fuels, and the generation of toxic chemicals. New green technology, however, may overcome these challenges:
Battery metals: Lithium and cobalt
Separation and purification techniques for battery critical metals such as lithium and cobalt tend to include brine evaporation and concentration, selective precipitation, and electrolysis or redox chemistry. Recently, researchers reported a new reagent to selectively bind lithium chloride out of an array of metal salts, including sodium, magnesium, and zinc with high efficiency. Precipitation can be a low-cost and scalable method, and it was successfully used to separate cobalt from nickel with the simple addition of carbonate, which causes the resulting cobalt complex to crash out leaving the nickel solvated.
Platinum group metals (PGMs)
For platinum group and rare earth elements, new technologies include fractional crystallization, solvent extraction cascades, ion chromatography, and selective precipitation. For example, a platinum complex was separated from other PGMs including palladium and rhodium with an organic additive called cucurbit[6]uril, which selectively binds to the platinum species and precipitates it out of solution.
Specialty metals: Gold and tellurium
Purifying gold ore is a toxic process and remains a challenge. For this reason, researchers found a way to strip gold anions from activated carbon using a relatively low-cost organic coordinating agent, α-cyclodextrin; the activated carbon is a typical substrate used in the gold industry.
Tellurium is a notoriously difficult critical metal for two reasons: its abundance is so low that it is rarely mined but rather separated from the waste of nickel mining, and it has several oxidation states that can be challenging to chemically activate for precipitation purposes due to its affinity to retain as an oxide. Researchers found, however, by adding certain catecholate derivatives to TeO2, they could kick out the oxygen and form a tellurium complex under redox conditions, successfully purifying the products.
We examined the CAS Content CollectionTM, the largest human-curated repository of scientific information, to better understand how separation and purification technologies are evolving. We found a strong upward trajectory for journal and patent publications related to these processes over the last 25 years (see Figure 3).

We also examined the number of journals and patents for select critical metals (see Figure 4). The overall growth reflects the rising trend in battery-operated devices such as mobile phones and, more recently, EVs. Lithium rises to be the top of published critical metals from 2018 onwards, surpassing cobalt in 2022. Out of the PGMs, platinum and palladium are the top reported metals in patents, followed by silver and gold, then rhodium and ruthenium. For journal articles, REEs such as cerium, neodymium, and lanthanum are the next most published after palladium.
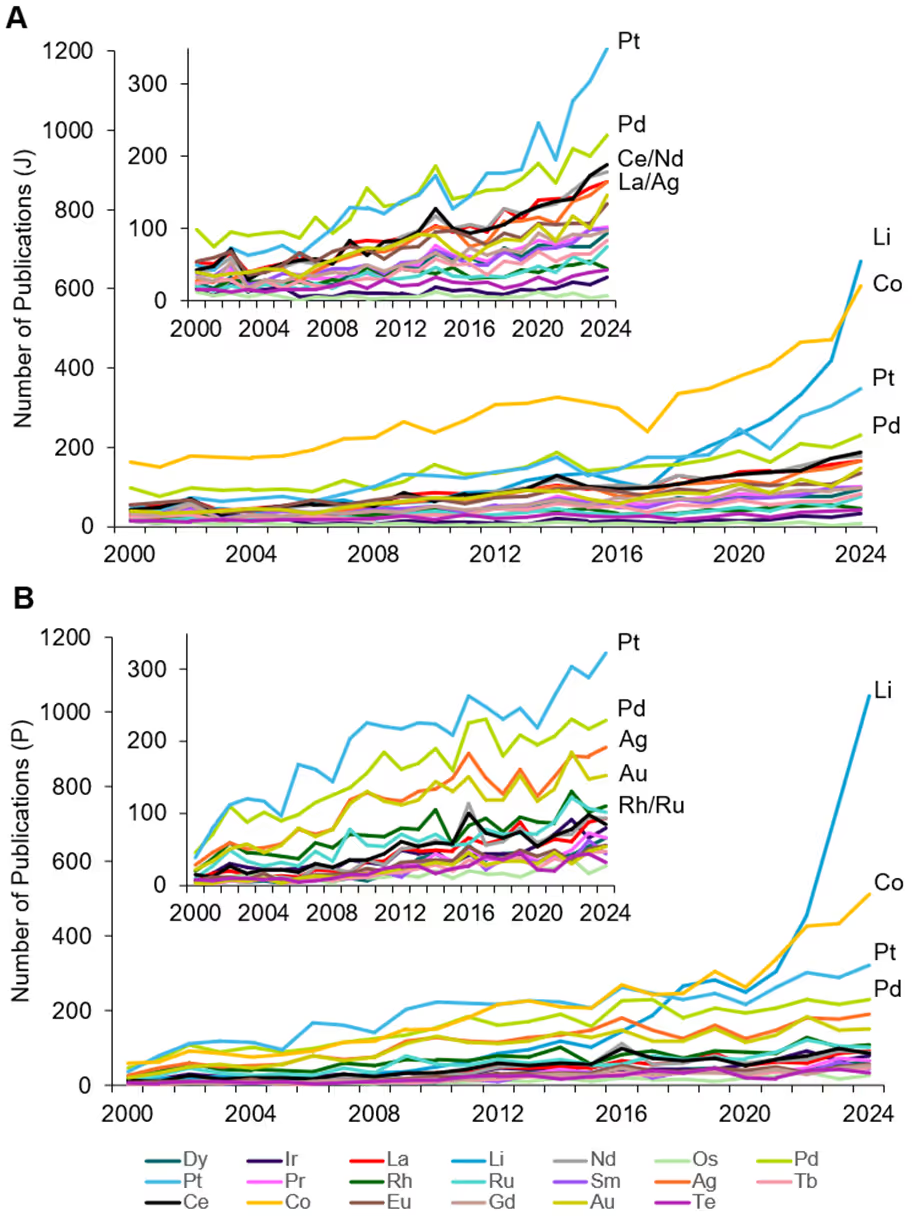
From this analysis, we can see the robust research activity around these elements, and the growth in patent publications seen in Figure 3 demonstrates that improved separation and purification technologies are reaching commercialization.
There is geographical differentiation around publication trends as well, encompassing both journals and patents. Figure 5 shows a heat map of the total documents as a function of critical metal and country, and grouped according to typical applications, such as batteries and solar, catalysis, and electronics. China is the top country for publications for many critical metals, particularly lithium, cobalt, gold, silver, platinum, palladium, lanthanum, cerium, and neodymium. Japan is a close contender for cobalt, platinum, and palladium. It is notable that there are fewer publications of tellurium and iridium, with China and Japan having the most, while osmium has the fewest publications out of all elements and countries or regions.
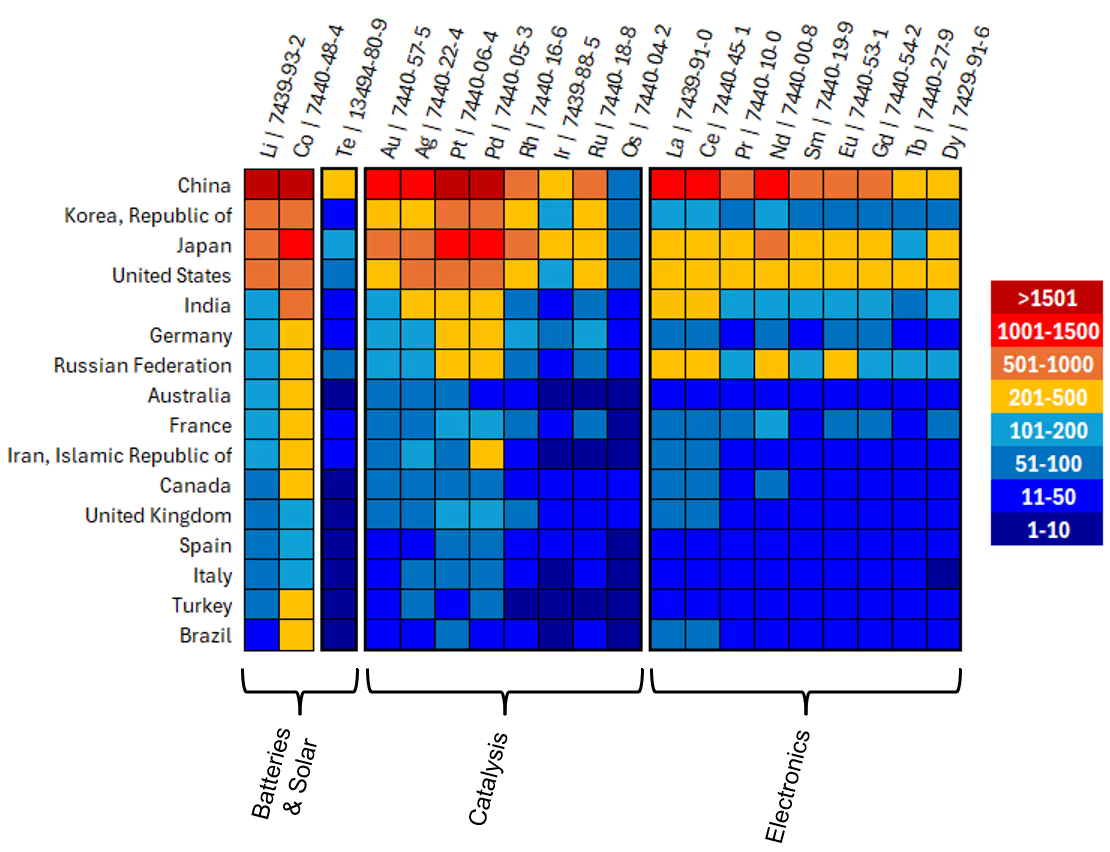
Two observations are made when comparing Figure 5 with Table 1: (1) China has both the most abundant CMs resources and publishes the most documents; (2) neither South Korea nor Japan have CM resources, but are in the top two and three countries or regions for documents published. This is inspirational for countries around the world in a similar position that are looking for technological growth in the face of relative scarcity.
For a more detailed understanding of the top patent holders, the top four countries and regions were selected, and the patent assignees were analyzed as a function of the CM elements (see Figure 6).
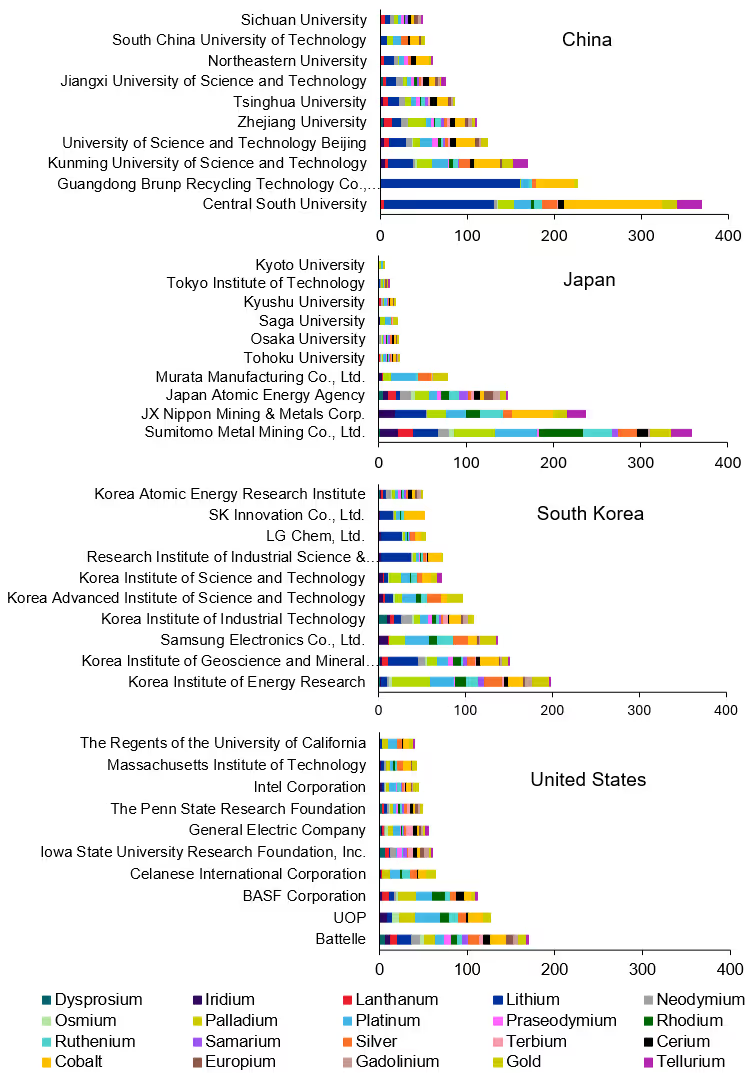
Central South University is the leading patent publisher for overall patents and cobalt patents, with Guangdong Brunp Recycling Technology Co. holding the most lithium patents. In Japan, the company Sumitomo Metal Mining Co. Ltd. is the largest patent holder, followed by JX Nippon Mining & Metals Corp., both widely published across many selected critical metals. In South Korea, palladium is the most patented critical metal, particularly by the Korea Institute of Energy Research. These analyses highlight the concentration of critical metals in Asia and research efforts centered there.
Critical metals, critical future
The critical metals market represents a substantial and growing sector of the global economy, with significant variations in market valuations across different metal categories. The lithium market is projected to reach USD $11.1 billion this year, while the cobalt market demonstrated continued growth driven by increased battery demand for electric vehicles and electronics. It’s now valued at USD $17 billion. The PGM market was valued at USD $21.6 billion in 2024 with expectations to grow to USD $22.7 billion in 2025, and the REE market shows varying valuations depending on the source, with estimates ranging from around USD $4 billion to over $8 billion in 2025, reflecting the market's complexity and rapid evolution.
These metals will remain indispensable to modern economies as long as they’re powering smartphones, computers, EVs, and renewable energy systems. Geopolitical tensions and trade policies may aggravate price volatility and supply-demand imbalances, so companies must develop robust sourcing strategies and comprehensive supply chain mapping to navigate this uncertain landscape.
The trajectory toward more sustainable extraction and separation technologies can assist with these challenges, with continued research investment likely to yield breakthroughs that reduce environmental impact while improving efficiency. The increase in scientific publications and patents suggests promising innovations that could transform traditional processing methods.
By extracting more critical metals with greater environmental sustainability, more of these vitally important elements will be available to power the electronic devices that modern life relies on. Fortunately, the outlook is bright for new and better methodologies, and that means a positive future for the technological engines of today as well as tomorrow.







