Drug Discovery
AI in drug discovery: Moving from potential to practical
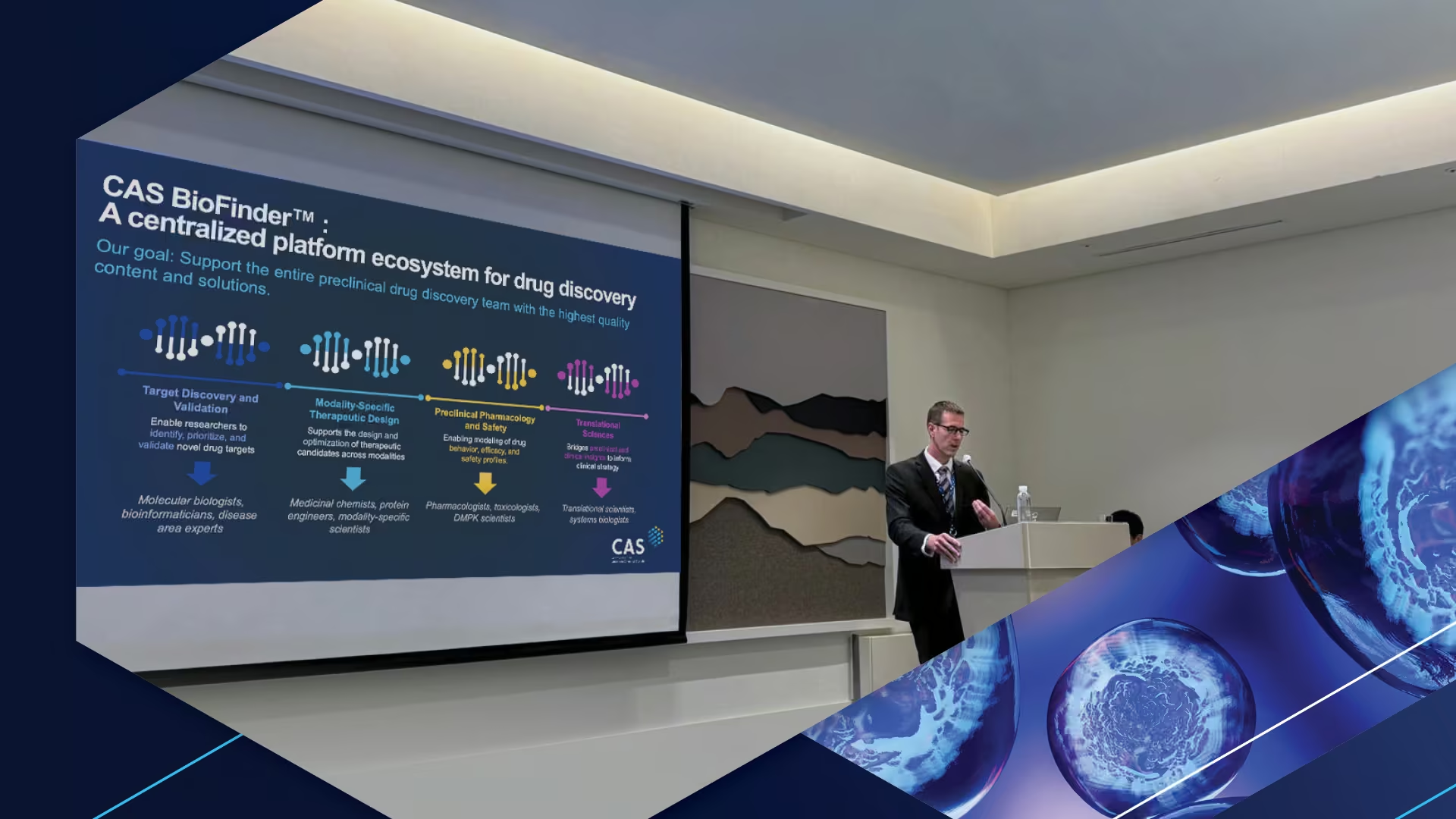
CAS hosted Life Sciences Summits across three regions in 2025, exploring practical AI integration in drug discovery. Learn about data governance, implementation barriers, and modality-specific challenges.
Read the reportRead the articleDownload the summarySee the infographicRead the publicationRead the recapWatch the video