
Move from search to solution confidently
Accelerate your next discovery with a scientist-trained, AI-enhanced, comprehensive knowledge platform designed to drive innovation across research, synthesis, and patent workflows.

Fragmented research slows innovation
Overlooked, irrelevant, or hard-to-find information inhibits discovery, increases risks, and leads to missed opportunities. CAS SciFinder® improves how you search, filter, and apply scientific information in the following ways:
- Find the most relevant answers quickly.
- Make confident, evidence-based decisions.
- Accelerate synthesis, patent, and research workflows.
Discover how CAS SciFinder helps researchers move from questions to breakthroughs faster.
Does your chemistry research have a solid foundation?
When reaction conditions, substance properties, or reference details are unclear, your entire workflow can fall apart. In chemistry R&D, every decision hinges on having the right information at the right time.
With access to the most comprehensive and up-to-date chemical data, you can:
- Plan and optimize synthesis routes confidently.
- Identify accurate substance and reaction details.
- Reduce costly lab rework.
See how CAS SciFinder can support your chemistry R&D team where it counts.
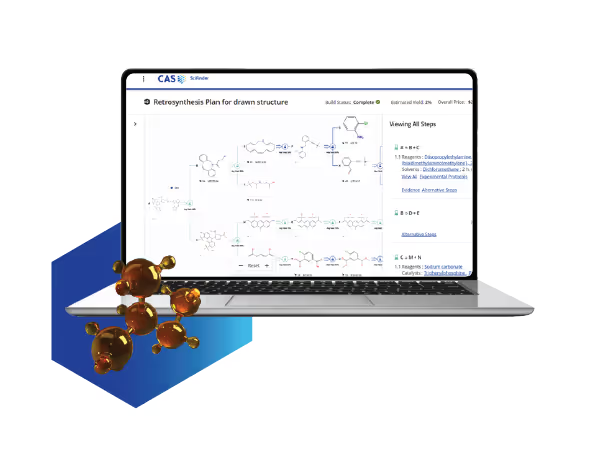
Retrosynthesis planning shouldn’t slow you down
When your synthesis planning involves incomplete data and predictions, you risk wasting time on inefficient routes. That means more delays, more rework, and less time for your desired outcomes.
With real-time retrosynthesis in CAS SciFinder, you can:
- Build synthesis plans based on expert-curated, real-world chemistry.
- Explore alternative steps, reagents, and conditions with full transparency.
- Tailor your route to your goals, whether that’s cost, selectivity, or speed.
"Using CAS SciFinder, I'm able to get key information about a variety of topics quickly and easily. This saves time and improves ideation."
AI is only as good as the data behind it
Most predictive tools rely on unverified or incomplete data, leading to unreliable results. CAS SciFinder is different. Its science-smart AI is trained on the world’s most trusted chemical content, expertly curated by scientists, not unceremoniously scraped from the web.
With this foundation, you can:
- Uncover relevant reactions, reagents, and conditions.
- Make informed decisions backed by transparent, evidence-based insights.
- Predict synthesis routes with greater accuracy and confidence.
Find out how our AI capabilities help you search smarter, faster, and with more relevant insights than ever before.
Academic research demands more than a basic search
In academic environments, time is limited, and expectations are high. Whether you're preparing coursework, conducting lab research, or writing a dissertation, finding accurate, relevant information quickly is essential. With CAS SciFinder, you can:
- Access trusted chemical literature, substances, and reactions in one place.
- Save time using natural language search to find expertly indexed literature and trusted substance data.
- Strengthen your research and learning with reliable, evidence-based insights.
See how students and faculty use CAS SciFinder to support discovery, teaching, and academic success.

Frequently asked questions
What is the CAS SciFinder Discovery Platform?
The CAS SciFinder Discovery Platform is a group of scientific search and analysis tools designed to enhance your scientific searches, academic research, retrosynthesis plans, analytical methods searches, formulation design, and regulatory compliance.
What is CAS SciFinder?
CAS SciFinder is the primary solution in the CAS SciFinder Discovery Platform. It allows users to search scientific literature, substances, reactions, chemical suppliers, and more with constantly enhanced features.
How much does CAS SciFinder cost?
Contact us for more details on an enterprise license and how we can fine-tune a plan that best suits your organization’s needs.
Does CAS SciFinder use AI?
CAS SciFinder uses science-smart AI to interpret your natural language queries accurately, giving you relevant answers faster without the need for creating complex or Boolean language searches.
How do I sign up for CAS SciFinder?
To sign up for CAS SciFinder, please request a demo.
How do I use CAS SciFinder?
For guides and tutorials on how to use CAS SciFinder, visit the CAS SciFinder Training section of CAS.org.
What is included in the CAS SciFinder Discovery Platform?
The CAS SciFinder Discovery Platform includes the following solutions:
CAS SciFinder - a solution for scientific, academic, and chemistry research
CAS Formulus - a solution for designing, searching, and managing formulations
CAS Analytical Methods - a solution for searching existing analytical methods
Related articles


Brazilian company resolves critical logistical challenge with CAS SciFinder Discovery Platform™
.avif)


