Gain new perspectives for faster progress directly to your inbox.
The World Health Organization (WHO) defines a microorganism that is not killed/inactivated after the due course of treatment as ‘resistant’, and the rise in resistant organisms is multifactorial. According to the Centers for Disease Control and Prevention (CDC), more than 2.8 million antibiotic-resistant bacterial infections occur each year, resulting in 35,000+ deaths. Worryingly, projections made by the World Bank estimate this number might increase to 10 million deaths a year by 2050.
As such, the WHO has declared antimicrobial resistance as one of the top ten primary health concerns, and new solutions are urgently needed.
Antibiotic resistance is multifactorial
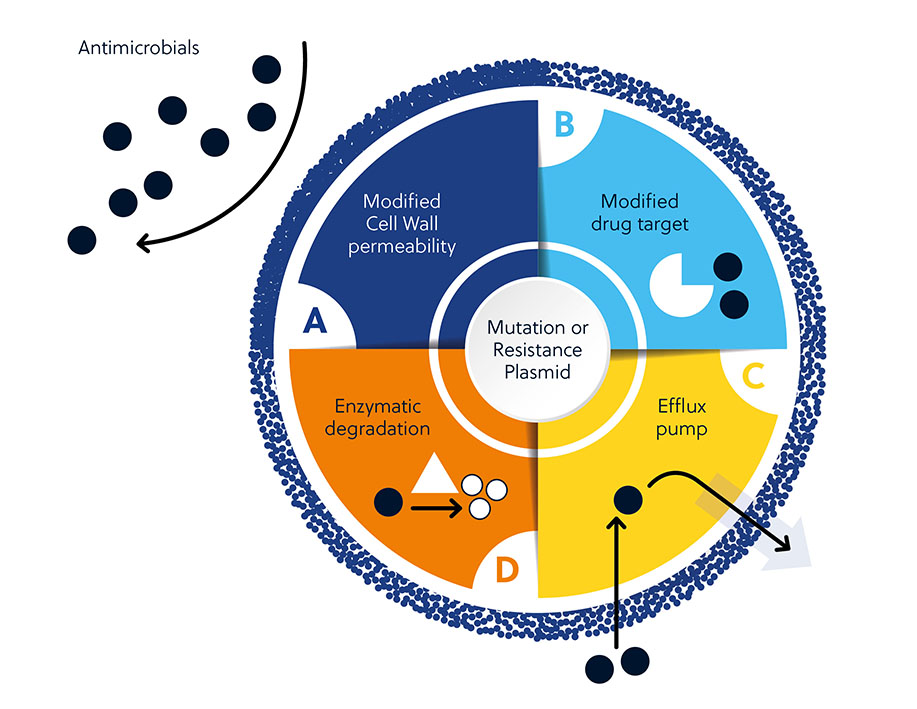
Antibiotic resistance can result from both intrinsic and acquired factors. Intrinsic factors include cell wall permeability, modified drug targets, activation of efflux pumps, and enzymatic degradation of antibiotics. Acquired resistance arises from the gain of new genetic material or mutation in the bacterial genome that mediates survival.
The need for new antibacterial treatments
Antibiotics cover many different classes. Each one is categorized by its structure and how it tackles bacteria in the body.
| Antibiotic class | Structure |
| Aminoglycosides (e.g., streptomycin, 57-92-1) | |
| Beta-lactams (e.g., penicillin, 61-33-6) | 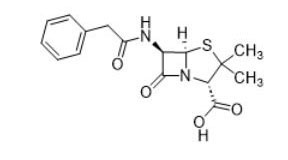 |
| Sulfonamides (e.g., sulfadiazine, 68-35-9) | 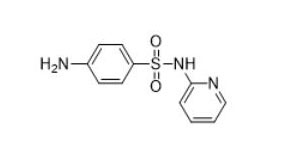 |
| Amphenicols (e.g., chloramphenicol, 56-75-7) | |
| Polymyxins (e.g., polymixin B, 1404-26-8) | |
| Tetracyclines (e.g., tetracycline, 60-54-8) | |
| Macrolides (e.g., clarithromycin, 81103-11-9) | |
| Pyrimidines (e.g., sulfadiazine 68-35-9) | 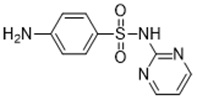 |
| Rifamycins (e.g., rifampicin, 13292-46-1) | |
| Quinolones and fluoroquinolones (e.g., nalidixic acid, 389-08-2) | |
| Streptogramins (e.g., quinupristin, 120138-50-3) | |
| Lincosamides (e.g., lincomycin, 154-21-2) | |
| Pleuromutilins (e.g., lefamulin, 1061337-51-6) | |
| Oxazolidinones (e.g., linezolid, 165800-03-3) |
Table 1. The different classes of antibiotics.
Despite these established treatment options, many infections are becoming resistant to existing antibiotic treatments. Coupled with an estimated rise in related deaths, there is a pressing need to rethink how we tackle bacterial infections.
The challenges new antimicrobials face
While the rise in antimicrobial resistance is multifaceted, it is compounded by the slower pace of developing new treatment options compared to the rate of antimicrobial resistance development.
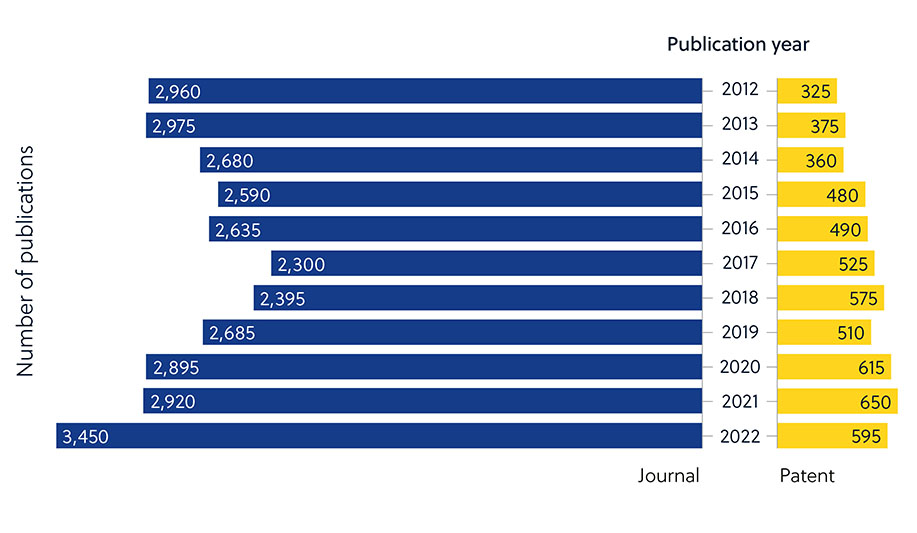
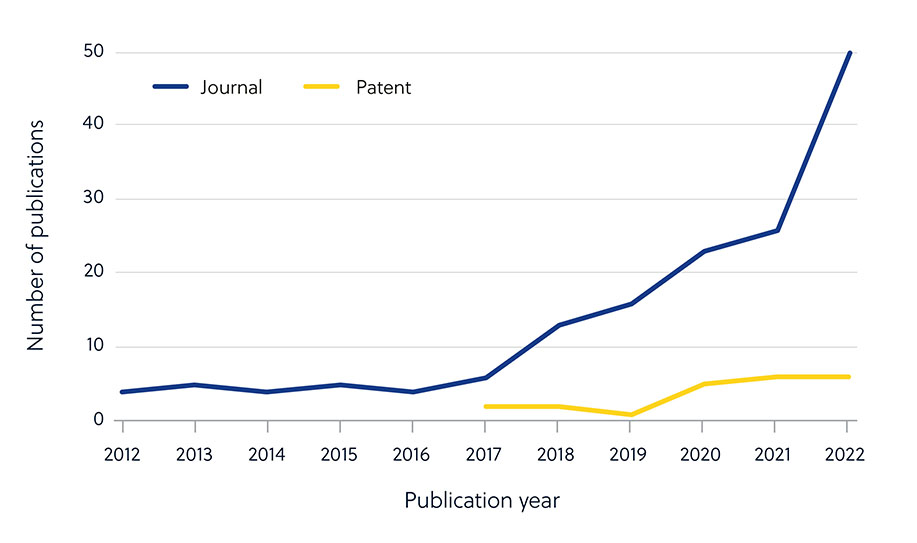
This is apparent when looking at the number of journal publications around antimicrobial resistance vs. the low proportion of patents (Figure 2). This indicates that researchers in academia are taking a more prominent role in developing new antimicrobials — and that these efforts must be translated into commercially available therapies.
This can be explained by several factors that make antimicrobial development challenging. Beyond many innate or acquired mechanisms microbes can use to resist antimicrobials (Figure 1), there are also broader factors that make development difficult (Figure 3).
The ability of bacteria to tolerate antimicrobials, combined with the high development cost and long timeframes (Figure 3), has led to few antibiotics reaching the market in recent decades despite the dire need.
Biofilms
Growing in a biofilm, or a layer of cells, allows bacteria to withstand antibiotic penetration. Biofilms can grow on catheters, pacemakers, joint prostheses, dentures, contact lenses, prosthetic heart valves, and implants. Growing in a biofilm, or a layer of cells, allows bacteria to withstand antibiotic penetration. Biofilms can grow on catheters, pacemakers, joint prostheses, dentures, contact lenses, prosthetic heart valves, and implants.
Outer layer of Gram-negative bacteria
Gram-negative bacteria are naturally resistant to various drugs that affect Gram-positive species due to their bilayer, outer membrane that is impenetrable to many drugs.
Return on investment
Low success rate of candidate molecules in combination with lesser return on investment are major challenges.
Timelines
It can take 10-15 years between initial molecule discovery to having a viable antibiotic reach the market.
Figure 3. Factors that can confound antimicrobial development.
Alternatives to conventional antibiotics
Bringing new antibiotics to market is a time-consuming challenge (Figure 3), so alternatives are helping combat antimicrobial resistance.
| Stringent response inhibition | Long-term survival of bacteria in the host, often asymptomatically, can lead to reactivation and reinfection. These long-surviving bacteria are called “persister bacteria.” Stringent response is a mechanism through which bacteria counter extreme starvation, which is thought to contribute to the development of persistent infection. Inhibiting this process could lead to greater bacterial susceptibility to antibiotics. |
| Bacterial vaccines | Preventing bacterial infections through vaccines leads to decreased antibiotic consumption and is likely to help with antibiotic resistance. A 2021 WHO report provided details of >60 and >90 vaccines in clinical and preclinical development, respectively. |
| Antimicrobial peptides | Antimicrobial peptides are gaining popularity as alternatives to small-molecule antibiotics. They are typically short (<100 amino acids) peptides with a broad spectrum of antimicrobial activity. According to the Antimicrobial Peptide Database, there are over 3,000 antimicrobial peptides as of November 2022. |
| Glycopeptides | Glycopeptides display antibacterial activity primarily against Gram-positive bacteria by inhibiting cell wall biosynthesis. Commonly used drugs in this group are vancomycin, teicoplanin, telavancin, dalbavancin, and oritavancin, but many new options are being developed, studied, and optimized. |
| Lipopeptides and lipoglycopeptides | Daptomycin is the only lipopeptide currently used against Gram-positive bacteria and functions by disrupting the bacterial cell membrane. The antibacterial effect observed appears dependent on the presence of and binding with calcium. Due to their large size, they are poorly absorbed when taken orally and tend to be administered intravenously. |
| Bacteriophages | Bacteriophages are viruses capable of infecting the bacteria cell and killing them by injecting viral DNA. The virus replicates within the cell and causes cell lysis as the replicas are released to find a new bacterial cell to infect. However, challenges still must be addressed to make bacteriophage therapy more viable, including poor in vivo efficacy for targeting bacterial species in the gut following oral administration. |
Table 2. Alternatives to conventional antibiotics
The future of antimicrobials
Enhanced drug delivery methods through materials can provide localized, prolonged, and stimulus-dependent antibacterial activity. There are several ways of achieving antimicrobial delivery outside of traditional administration. Medical devices such as implants and catheters can be infection sources, which can be potentially prevented by using antimicrobial materials. Likewise, antimicrobial coatings on high-traffic surfaces can reduce the transmission of microbes and minimize the need for cleaning.
Hydrogels
Used together with antibiotics for drug delivery and wound healing. Hydrogels act in situ while being exposed to bodily cells and fluids to enable diffusible or gel-bound antibiotic agents such as antimicrobial peptides to be administered.
Nanoparticles
The small size of nanoparticles makes it easy for them to deliver drugs effectively. Surface modification can be used to tailor them for specific targets and locations, and the surface chemistry and composition control the timing of activity, drug release, and duration of action.
Composites
Composites use multiple materials together and are used for medical devices such as dental implants. The variety of materials capable of exerting antimicrobial activity have proven efficacy in both treating and preventing infection and transmission.
Films or coatings
Reducing the ability of medical devices to transmit infection would be an effective way to improve the health and survival of hospital patients. The ability to harness UV or visible light and generate reactive oxygen species that prevent microorganisms from adhering are two examples of effective films and coatings.
Scaffolds and implants
Useful for wound and bone healing, these typically have a high surface area-to-volume ratio and are more persistent than hydrogels. Implants with cationic polymers, copper nanoparticles, or nitric oxide-releasing agents have shown promising efficacy.
Figure 4. Materials and solutions that can reduce antibiotic usage
Advances in artificial intelligence (AI) have led to an acceleration in antimicrobial drug development using algorithms to identify potential new molecules. Although the number of journal publications has steadily increased, there hasn’t been a corresponding surge in patent applications, suggesting that most antimicrobial AI research is still in the academic stage (Figure 5).
The rise in multi-drug-resistant bacteria poses an alarming threat to human health, and the need to develop novel antibiotics and antibacterial materials is urgent. Widespread AI use is still in its infancy; however, it holds promise for streamlining and reducing timelines for future efforts. Learn more about AI’s impact on chemistry in our Insight Report, the rise of large-language models, and how biomaterials are being used across the therapeutic landscape in a variety of new approaches.