キメラ抗原受容体発現T細胞(CAR-T)の抗腫瘍免疫学への応用は、過去10年間で大きな進歩を遂げてきました。しかし、これらの治療法の広範な採用と実施には、主に移植片対宿主病(GvHD)や腫瘍関連免疫抑制という課題が依然として残っています。これにより研究者たちは、抗腫瘍免疫において重要な役割を担う可能性のある特殊なTリンパ球のサブセットである「非従来型T細胞」を探求するようになりました。
ヒトの粘膜関連不変T細胞(MAIT細胞)は、そのような非従来型T細胞の一種です。これらはさまざまながん、細菌・ウイルス感染症、自己免疫疾患、炎症性疾患、代謝性疾患に大きな影響を及ぼすことが示されています。MAIT細胞は他の非従来型T細胞と比べて比較的新しい発見であり、がんやその他の疾患治療への応用は、個別化医療においてゲームチェンジャーとなる可能性があります。研究者たちは、個別化されたCAR-T細胞採取や再注入といった高額な手法の代わりに、同種異系のMAIT細胞を「off-the-shelf(汎用型)」の免疫療法として活用する方向で研究を進めています。
他のT細胞とは異なるMAIT細胞の機能
MAIT細胞は主に肺や腸といった粘膜組織に存在しますが、皮膚、脂肪組織、肝臓にも存在します。通常のT細胞とは異なり、MAIT細胞は主要組織適合遺伝子複合体(MHC)分子によって制限されませんが、MHC関連タンパク質1(MR1)を認識します。これらは半不変的なTCR α鎖(ヒトではVα7.2-Jα33/20/12)と、限定的な範囲のTCR-β鎖(主にTRBV20およびTRBV6遺伝子ファミリー由来)を発現します。
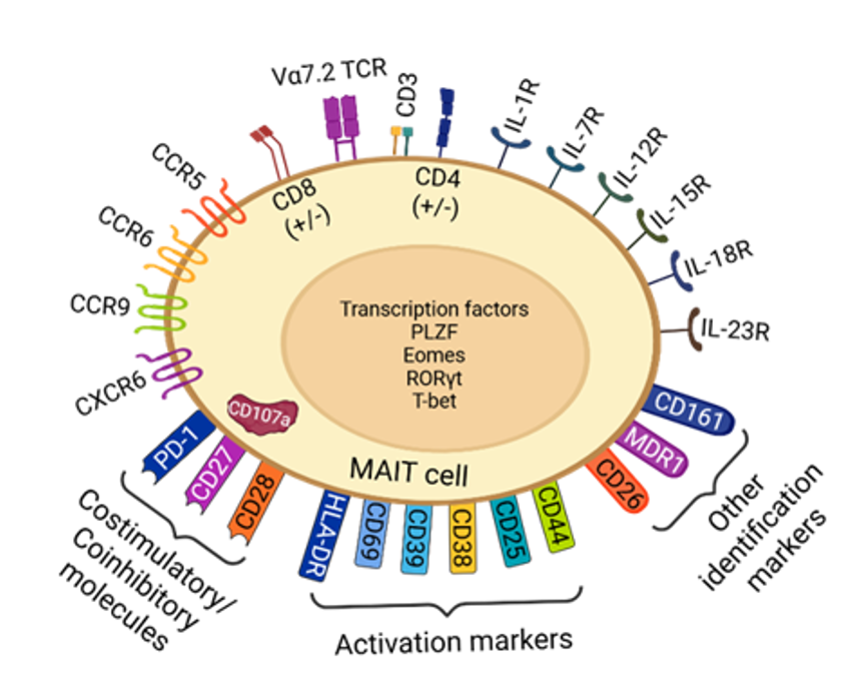
健康な成人におけるMAIT細胞のおよそ80%はCD8を発現していますが、15%はダブルネガティブ(CD4-CD8-)であり、5%未満がCD4またはダブルポジティブ(CD4+CD8+)を発現しています。図1に示すように、MAIT細胞は識別、活性化、共刺激、共抑制、免疫療法標的分子PD-1といった多数のバイオマーカーを含んでいます。
MAIT細胞は、T細胞受容体(TCR)依存性経路と非依存性経路の2つの異なる経路で活性化されます(図2参照)。TCR依存性経路は、5-OE-RUや5-OP-RUなどの抗原がMR1細胞上で提示されることで始まります(MR1細胞には、樹状細胞、マクロファージ、B細胞といった抗原提示細胞(APC)が含まれる場合があります)。この結合によりMAIT細胞が活性化し、CD25、CD69、CD38、HLA-DRなどのマーカーの発現上昇や、TNF-αおよび/またはIL-17の産生が起こります。
一方で、IL-7、IL-12、IL-15、IL-18といったサイトカインによっても、TCRを介さずにMAIT細胞が活性化されます。TCR依存性の活性化では、非依存性経路と比べて炎症性サイトカインの産生が多くなります。いずれの経路もCD107aによる脱顆粒や、標的細胞へのグランザイムA、B、K、H、Mおよびパーフォリンの放出を誘発します。

当社は、人間がキュレーションした世界最大規模の科学情報のリポジトリであるCASコンテンツコレクションTMを分析し、MAIT細胞の治療的可能性を調査する研究が2016年以降急増していることを発見しました。これらの研究は、がん、自己免疫疾患、免疫療法、移植後のシナリオや慢性感染症を対象としています。MAIT細胞に関する特許の動向も、過去5年間で急速に拡大しています(図3参照)。


この急激な成長は、MAIT細胞療法が商業化に向けて進展していることを示唆しています。たとえば、注目すべき特許出願(CN118853585A)では、がん、感染症、自己免疫疾患の治療におけるCARを発現するMAIT細胞が記載されています。このイノベーションは、治療対象となる患者に対して同種異系のMAIT細胞がCARを発現するというもので、標的療法における重要なブレークスルーです。MAIT細胞がドナー由来で開発され、患者ごとに作製する必要がなくなれば、より多くの患者に低コストで治療を拡大できる可能性があります。
がんおよびその他の領域におけるMAIT細胞治療の可能性
CASコンテンツコレクションにおいてMAIT細胞に関連する主要概念を分析したところ、がんが最も広く研究されており、文献のほぼ半数を占めていました(図4参照)。主ながん種は、血液がん、消化管がん、肺がん、婦人科がん、皮膚がんです。がん以外にも、MAIT細胞は炎症性疾患(15%)、自己免疫疾患(13%)、ウイルス性疾患(9%)、細菌感染症(6%)、その他肺疾患、肝疾患、代謝障害、免疫疾患、血液疾患、線維症などで研究されています。これらの洞察は、MAIT細胞が多様な疾患において果たす役割と治療標的としての可能性を強調しています。

がん免疫療法におけるMAIT細胞
MAIT細胞は、CAR、TCR、NKRを介した細胞毒性といった多様な腫瘍標的化メカニズムを用いて、多くの種類の腫瘍細胞を認識・破壊する一方で、腫瘍抗原の回避リスクを低減します。さらに、これまでアロ反応性の有害事象が示されていないため、MAIT細胞は将来的にoff-the-shelfの養子がん免疫療法において主要な細胞タイプとなり得る可能性があり、ヒト白血球抗原(HLA)の不一致という障壁を克服できるかもしれません。
がん免疫療法において有望なMAIT細胞の独自の特性と機能は以下のとおりです。
- 複数の腫瘍標的化メカニズム:MAIT細胞は以下の経路で腫瘍を攻撃できます。
- 遺伝子工学の可能性:MAIT細胞はサイトカイン工学やチェックポイント遺伝子ノックアウトといった遺伝子改変に適応可能であり、その機能的特性を強化できます。これには、持続性の延長、細胞毒性の向上、腫瘍微小環境における免疫抑制への耐性強化が含まれます。
- 移植片対宿主病(GvHD)の改善:MAIT細胞はMR1によって制約され、HLA不一致分子や自己抗原タンパク質を認識しません。そのため、MAIT細胞がGvHDを引き起こすことはないと考えられます。前臨床マウスモデルでは、CD19 CAR-T細胞とは対照的に、CD19 CAR-MAIT細胞はGvHDを引き起こすことなく安全に生着しました。
- 抗がん剤耐性:MAIT細胞は他のT細胞サブタイプと比較して化学療法に対する耐性が高く、さまざまな化学療法薬を迅速に排出する能力を持ちます。この耐性は、自家CAR-T細胞療法の開発においてMAIT細胞が有望な代替細胞源であることを示唆しています。というのも、現在、自家CAR-T細胞療法を受ける適格患者は、特に化学療法を含む複数の一次治療を受けている必要があるためです。
- 抗PD-1/PD-L1免疫療法の標的となる可能性:抗PD-1療法のような免疫チェックポイント阻害薬(ICI)は、多くの悪性疾患において顕著な治療効果を上げています。MAIT細胞は多数のICI標的を発現しており、ICI免疫療法における重要性を示しています。in vivo研究の報告によると、抗PD-L1療法とMAIT細胞の活性化を組み合わせた場合、野生型マウスでは腫瘍の成長が抑制されましたが、Mr1-/-マウスでは効果が見られませんでした。これは、ICIとex vivoで活性化されたMAIT細胞を組み合わせた併用療法が、抗PD-1療法などのICI治療効果を高める新しい戦略となり得ることを示しています。
- 予後マーカー:がん患者において、ベースラインおよび抗PD-1療法後にMAIT細胞の頻度が増加していることが確認されました。これは、多発性骨髄腫やメラノーマの患者で抗PD-1療法への良好な反応と相関していました。したがって、MAIT細胞の存在や頻度は、特定のがん治療に対する患者の反応を予測するバイオマーカーとして機能する可能性があります。
免疫介在性炎症性疾患におけるMAIT細胞
自己免疫疾患においては、MAIT細胞が主に疾患の病態に寄与しており、これらを選択的に抑制することが有望な治療アプローチとなります。研究者は、MAIT細胞の強力な阻害リガンドとして機能するアセチル-6-ホルミルプテリンなどの化合物を特定しています。このような標的型MAIT細胞阻害剤の開発は、自己免疫疾患において、MAIT細胞の過剰活性が炎症や組織損傷を引き起こす場合に、より精密な治療介入を可能にする製薬研究の機会を提供します。
MAIT細胞と抗ウイルス防御
最近の研究により、MAIT細胞は、ヒトおよびマウスモデルにおいて、主に抗原非依存的に、HIV-1、肝炎ウイルス、インフルエンザウイルス、SARS-CoV-2などさまざまなウイルス感染に反応できることが実証されています。疾患の状況に応じて、MAIT細胞は宿主に直接的または間接的な抗ウイルス保護を提供し、他の免疫細胞のリクルートを助ける可能性があります。しかし、状況によっては炎症や免疫病理を悪化させる場合もあります。さらに、慢性ウイルス感染はMAIT細胞の機能的・数的障害と関連しており、宿主防御に二次的な影響を及ぼす可能性があることを示唆しています。
有望なワクチン構成要素としてのMAIT細胞
MAIT細胞は、豊富に存在し、すでにエフェクター記憶型表現型を備え、粘膜部位に分布し、保存されたT細胞受容体を持つことから、ワクチン開発において独自の利点を提供します。マウス研究では、特定のリガンドと共刺激シグナルを用いることで、MAIT細胞集団を迅速に拡大し、呼吸器感染症に対する局所的免疫応答を強化できることが示されています。安定したMAIT細胞活性化化合物の開発は、従来のB細胞またはT細胞ワクチンに生物学的アジュバントとして追加されることで、予防ワクチンの大きな進歩につながる可能性があります。このアプローチは、MAIT細胞の迅速な応答能力と感染侵入部位での戦略的な配置を活用することで、ワクチンの有効性を向上させる可能性があります。安定したMAIT細胞リガンドに関する継続的な研究は、ワクチン技術における有望な方向性を示しています。
創傷治癒におけるMAIT細胞
MAIT細胞の創傷治癒における新たに発見された役割は、下肢潰瘍、褥瘡、熱傷などの慢性皮膚創傷を抱える患者にとって、有望な臨床応用の可能性を示しています。リボフラビンを産生する共生菌を創傷部位に導入し、MAIT細胞を自然に活性化する方法と、合成MAIT細胞活性化化合物を局所的に直接塗布する方法の2つの潜在的な治療戦略が浮上しています。後者のアプローチはマウス研究で成功を示しており、概念実証の妥当性が認められています。
この治療法は、その臨床実施経路が単純である点で魅力的です。多くのMAIT細胞リガンドは局所適用が可能で、安全性も確立されていることから、大規模臨床試験への迅速な移行が期待され、従来の治療では治癒に時間がかかっていた創傷の治療選択肢を変革する可能性があります。
MAIT細胞療法の次のステップ
CAR-MAIT細胞は前臨床研究で開発されており、その抗腫瘍活性の実現可能性を示すエビデンスがあります。しかし、がん治療への応用を探る臨床試験はまだ行われていません。必要な臨床試験の実施には時間がかかりますが、MAIT細胞がoff-the-shelfのCAR-T療法として機能する可能性は非常に有望です。バイオテクノロジー企業Pluriは、バル=イラン大学研究開発会社(BIRAD)と提携し、固形腫瘍治療を目的とした同種異系、off-the-shelf、胎盤由来のCAR-MAIT細胞を開発しており、画期的な治療法が近いうちに実現する可能性があることを示唆しています。
これまでのCAR-T療法は、各患者からT細胞を採取、改変、再注入する工程を必要としており、このプロセスは高額かつ時間がかかります。もしMAIT細胞があらゆる患者の腫瘍を攻撃できるように設計されれば、より多くの患者にこうした療法を提供できる大きな前進となるでしょう。