糖尿病は急速に、最も緊急性の高いグローバルな公衆衛生危機の一つになりつつあります。現在、世界中で5億4,000万人が糖尿病を患っており、その数は2045年までに7億8,300万人に増加すると予測されています。症例の90%以上はインスリン耐性とベータ細胞機能障害を含む2型糖尿病です。
1型糖尿病(若年性糖尿病)は、膵臓のインスリンを産生するベータ細胞が破壊される自己免疫疾患であり、驚くべき速度で増加しています。2021年時点では世界中で800万人以上が1型糖尿病を患っており、2040年までにその数は1,700万人にまで増加すると予測されています。
糖尿病は、放置していると複数の体組織に影響を及ぼす慢性合併症を引き起こす可能性があります。特に、網膜症、腎症、神経障害などの微小血管疾患や、心血管、脳血管、末梢血管疾患などの大血管疾患が含まれ、これらはすべて高い罹患率と死亡率を示しています。2021年、糖尿病および糖尿病に起因する腎臓病が世界中で200万人以上の死因となり、心血管疾患による死亡の約11%が高血糖に関連していました。
糖尿病研究における変化
1型糖尿病と2型糖尿病の両方を治療するための従来のアプローチには、血糖値の継続的なモニタリングと健康的なライフスタイルの維持が含まれます。1型糖尿病患者は、毎日の注射という形でインスリン補充を行わなければならず、2型の患者はメトホルミン、スルホニル尿素、DPP-4阻害薬などの経口薬を使用します。
これらの疾患の有病率の増加と世界人口の高齢化に伴い、2型糖尿病の症例が増加すると予想され、より良い治療オプションの必要性が示されています。2022年、FDAは1型糖尿病の発症を遅らせるteplizumabを承認し、2型糖尿病に対しては、GLP-1受容体作動薬やSGLT-2阻害薬などの新薬が検討されています。
しかし、最近の飛躍的進歩により、1型糖尿病患者のインスリン分泌を回復させる膵島細胞療法が登場しました。2000年代初頭、幹細胞をインスリン産生細胞に分化させる試みが初めて成功し、2010年代には臨床試験が始まりました。ただし、1型糖尿病患者向けの最初の細胞療法であるLantidraのFDAによる承認は、2023年まで待たなければなりませんでした。それでも、最近の研究や試験では、幹細胞由来の膵島細胞が糖尿病患者のインスリン産生を回復させ、インスリンの注射の必要性を減らすか、あるいはなくす可能性があることも示されました。
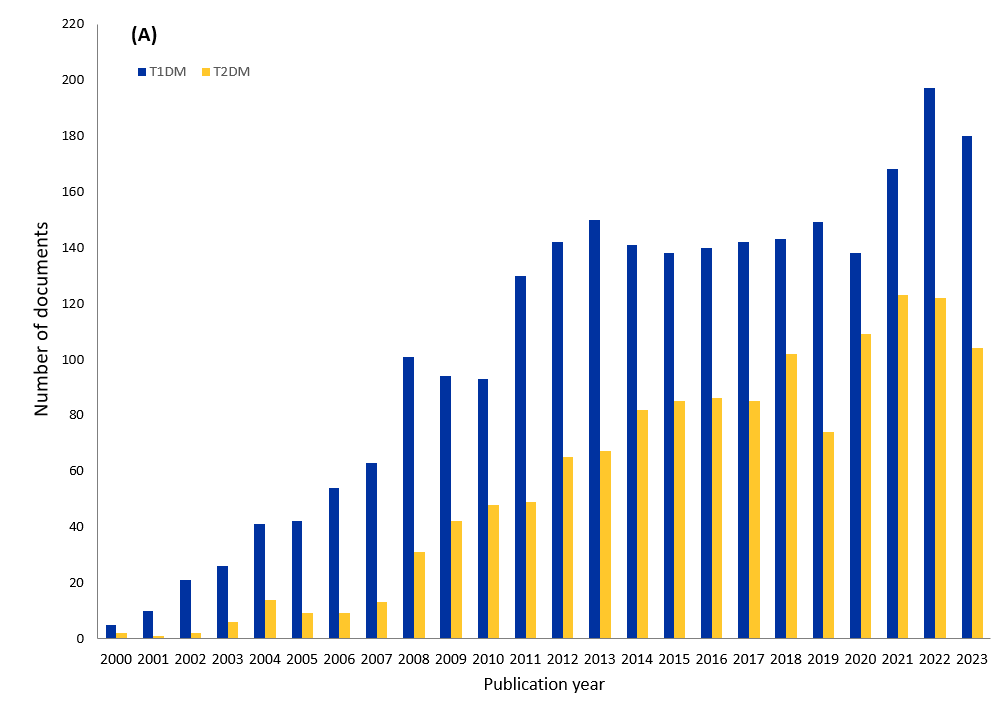
幹細胞治療を成功させ、その効果を長続きさせるための新しい戦略が模索されています。これらのアプローチは、1型糖尿病と2型糖尿病の治療効果がより永続的になる可能性があります。当社は、CASが収集した最大の科学情報のリポジトリであるCASコンテンツのコレクションTMを調査し、2000年から2023年にかけて糖尿病の細胞治療に関する出版物が著しく増加していることを見出しました。
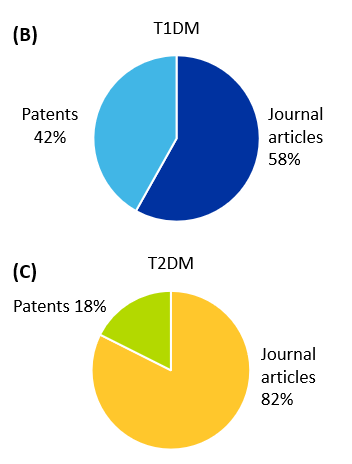
図1に示すように、幹細胞療法と1型糖尿病に関連する出版物は、2型糖尿病よりも多くなっています。さらに、1型糖尿病では、学術論文が全体の58%を占め、特許が42%を占めています。2型糖尿病では、学術論文の数は82%と高く、特許の数はわずか18%となっています。
糖尿病治療の可能性を示す幹細胞の種類
幹細胞とは、さまざまな特殊化した細胞タイプに分化できる未分化細胞のことです。これには主に次の3種類があります:
- 成体幹細胞:造血幹細胞(さまざまな種類の血液細胞に分化)や間葉系幹細胞(骨、軟骨、脂肪細胞に分化)など、限られた範囲の関連細胞に育つ可能性のある組織に見られる多能性細胞。
- 人工多能性幹細胞(iPS細胞):成体(成熟)体細胞から直接生成される多能性幹細胞の一種。これらの細胞は、胚のような状態に再プログラムされ、体内のどの細胞タイプにも分化できるようになります。
- 胚性幹細胞(ES細胞):初期段階の胚から得られる多能性細胞で、ほぼあらゆる種類の細胞に育てることができます。
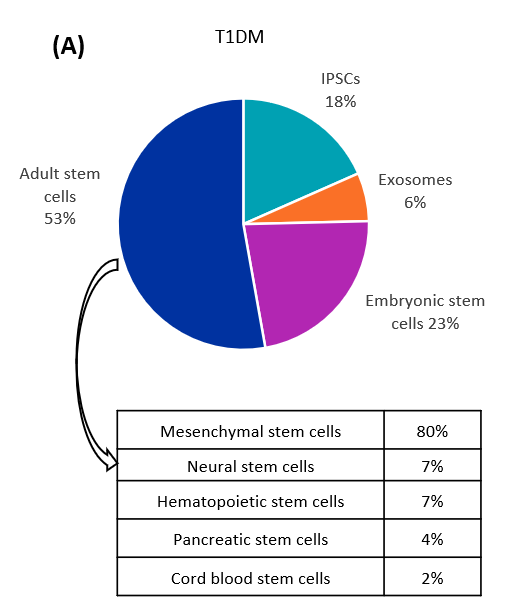
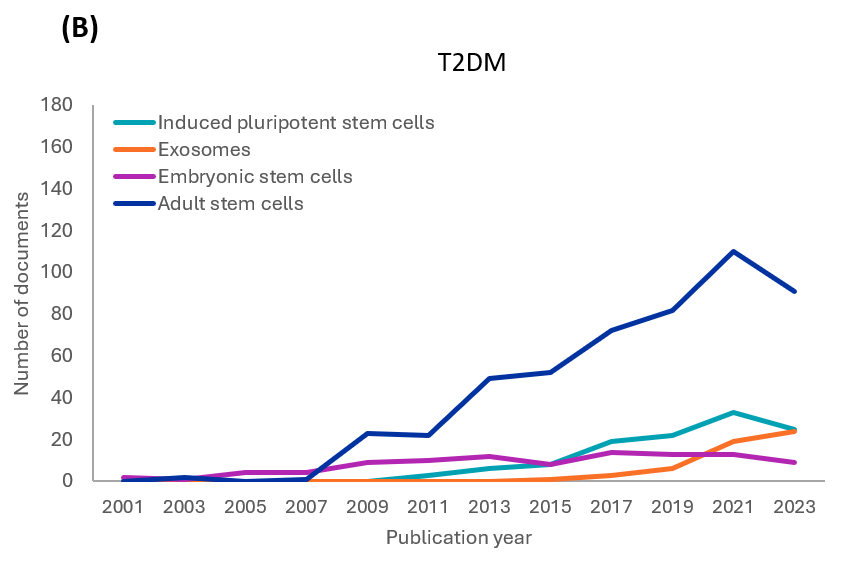
糖尿病治療の目的で研究されている幹細胞の種類を分析した結果、1型および2型糖尿病において、成体幹細胞が最も一貫して研究されていることが判明しました(図2参照)。成体幹細胞のなかで最も顕著なタイプは間葉系幹細胞(MS細胞)で、これに神経、造血、膵臓、臍帯血幹細胞が続きます。

ES細胞由来の治療薬候補VX-880は、第III相臨床試験(NCT04786262)の段階にあります。VX-880は、1型糖尿病の治療用に開発された、治験段階の同種幹細胞由来の、完全に分化したインスリン産生島細胞による治療法です。この治療薬候補については有望な結果が得られており、2024年11月にVertex Pharmaceuticalsが、50人の患者を対象とした重要な第III相試験の開始を発表しました。これは、多くの種類の幹細胞が、糖尿病を治療するだけでなく治癒する可能性を秘めていることを示しています。
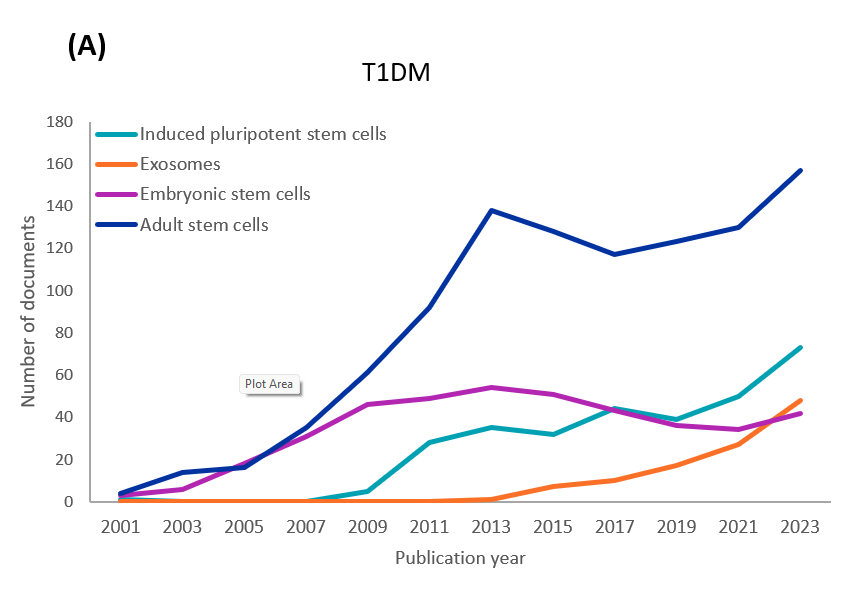
当社によるCASコンテンツのコレクションの分析では、1型および2型糖尿病研究に関連する各幹細胞の種類の出版物の年次傾向を確認しました(図3参照)。どちらの種類についても、初期の研究では成体幹細胞(主にMS細胞)と胚性幹細胞(ES細胞)が調査されました。ただし、成体幹細胞の研究はES細胞に比べて増加しています。iPS細胞に関連する文献も、両方の型で着実に増加しています。約10年前に始まったばかりのエクソソーム関連の文献や研究も増加しています。
さまざまな種類の幹細胞の利点と限界
- MS細胞:これらの成体幹細胞は、様々な供給源(骨髄、脂肪組織)から単離することができ、MHC-II複合体が少なく、MHC-I複合体の発現率が低いため、免疫原性が低く抑えられます。免疫反応や炎症を調節することができるため、腫瘍形成のリスクが少なく、臨床使用において比較的安全です。しかし、多能性幹細胞と比較すると、分化能には限界があり、ドナーによって細胞の質や有効性にばらつきが生じることがあります。また、in vitroで幹細胞状態を維持するのが難しく、拡張性に問題があります。最後に、幹細胞がヒトのドナーに由来する場合、倫理的な懸念が生じる可能性があります。
- iPS細胞:iPS細胞は体細胞に由来するため、倫理的な問題は少なく、患者本人由来のものであれば免疫拒絶反応を避けることができます。ただし、制約という点に目を向けると、これらの細胞の再プログラミングには時間とコストと困難さが伴います。また、再プログラミングの過程で遺伝子変異や腫瘍形成のリスクが高まる可能性もあります。
- ES細胞:これらの細胞は高い再生能力を示し、十分に特性が見極められ、研究されています。増殖率が高く、大量生産にも適しています。しかし、奇形腫形成のリスクがあり、その使用には倫理的な懸念があります。
各種幹細胞は重要な研究の対象となっています。表1をご覧ください。
| 幹細胞の種類 | 主要な研究の成果 |
|---|---|
|
MSCs |
MS細胞移植はHbA1cレベルを大幅に低下させ、1型患者の空腹時C-ペプチド・レベルを増加させます。 |
|
自家MS細胞治療は新規発症1型患者のβ細胞の機能を温存する可能性があります。 |
|
|
2型患者では、空腹時血糖値、HbA1c、インスリン必要量が減少し、空腹時のCペプチドとCペプチド/グルコース比が増加しました。 |
|
|
2型患者では、インスリン必要量が減少し、HbA1cがわずかに増加し、グルカゴン刺激Cペプチドが大幅に増加しました。 |
|
|
iPS細胞 |
iPS細胞をインスリン産生細胞に再プログラムし、患者に注射した結果、1型糖尿病患者においてインスリンの安定した産生が1年以上注射なしで維持されました。 |
|
iPS細胞はインスリン産生細胞に分化させることができるため、前臨床試験で有望視されています。 |
|
|
ES細胞 |
有望な結果が示されている現在進行中の臨床試験では、ES細胞由来の膵島細胞が患者に移植され、安全性と有効性のベンチマークを達成しました。 |
|
ES細胞由来のベータ細胞はインスリン産生を回復し、糖尿病マウスの血糖値コントロールを改善することができます。 |
|
|
幹細胞由来のエクソソーム |
MS細胞由来のエクソソームは、マウスを使用した1型糖尿病研究において、膵島の炎症を抑制し、疾患の進行を緩和することを示しました。 |
|
MS細胞由来のエクソソームは膵島構造を回復し、ラットにおける2型糖尿病の研究でインスリン感受性を高めました。 |
表1:幹細胞の種類と主要な研究からの知見。
幹細胞治療における進展と課題の展望
幹細胞治療は糖尿病に伴う多くの合併症の治療に使用される可能性があります。従来の糖尿病治療法は血糖値の調節に重点を置いていますが、糖尿病に伴う合併症の軽減や治療には効果的ではありませんでした。
例えば、最近のメタ分析では、糖尿病足病変の患者が幹細胞療法の恩恵を受けることがわかりました。治癒率、切断率、疼痛スコア、新血管形成率などのさまざまなパラメータがそのことを如実に示しています。他の研究では、糖尿病性腎症および糖尿病性心血管合併症における幹細胞のメカニズムと応用性を解明しようとする試みが行われています。
幹細胞は糖尿病治療において大きな可能性を秘めていますが、その広範な応用を妨げる可能性のあるいくつかの課題も残っています。特に懸念されるのは、細胞が別のドナー(同種供給源)からのものである場合の免疫拒絶反応です。移植された幹細胞の生存能力と効率を維持することも課題であり、腫瘍が発生するリスクもあります。移植後の免疫原性と催奇形性に対処するために、研究者たちは免疫分離装置、カプセル化法、または免疫抑制療法を使用しています。
研究者がこれらの課題に道筋をつけるにつれて、1型糖尿病と2型糖尿病の治療法だけでなく、治癒法も開発できる可能性があります。これらの病気が蔓延する中、科学コミュニティにとっては、革新的な治療法の選択肢でブレークスルーを起こし続けることが不可欠です。







