Over 100,000 people in the U.S. are on the waiting list for an organ transplant. In 2024, nearly 48,000 organ transplants were performed, and while that number represents impressive growth in these life-saving procedures, it’s still far below the overall need. As the global population ages, this mismatch between available organs and patients in need will continue to increase.
However, organs from animal donors may be the solution. Known as xenotransplantation, this procedure has a long history dating back to the early 20th century, but immunological issues and organ rejections prevented these transplants from being successful. With the advent of genetic editing technology, specifically CRISPR-Cas, researchers are increasingly able to address immuno-compatibility issues. They are getting closer to making hearts, kidneys, and other organs and tissues available for transplant from animal donors to human recipients, as seen in several breakthrough transplants between 2022-2024. These innovations could bring new hope to many thousands of patients in need of life-saving organs.
What is xenotransplantation?
According to the FDA, “Xenotransplantation is any procedure that involves the transplantation, implantation or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source, or (b) human body fluids, cells, tissues, or organs that have had ex vivo contact with live nonhuman animal cells, tissues or organs.”
Doctors began experimenting with xenotransplantation in the early 1900s, but it wasn’t until the middle of the century, when immunosuppressant drugs were developed, that transplantation success rates improved. The 1960 Nobel Prize in Physiology or Medicine was awarded to Peter Medawar for his pioneering work in transplantation immunity.
List of key events in the history of xenotransplantation
Presented as year, organ attempted to be transplanted, donor animals or event, and reference.
- 1906: Kidney, Pig and goat.
Rodger, D. and Hurst, D. J. Xenotransplantation 2022, 29, e12765 - 1910: Kidney, Macaque monkey
Unger, E. Berliner Klinische Wochenschrift. 1910, 47, 573-578 - 1913: Kidney, Monkey
Morel, L. and Papin, E. Biologie Médicale 1913, 11, 397 - 1923: Kidney, Lamb
Neuhof, H. D. The transplantation of tissues 1923, 260 - 1963: Kidney, Baboon
Hitchcock, C. et al. JAMA 1964, 189, 934-937 - 1963: Kidney, Rhesus monkey
Reemtsma, K. et al. Science 1964, 143, 700-702 - 1963: Heart, Chimpanzee
Hardy, J. et al. JAMA 1964, 188, 1132-1140 - 1964: Kidney, Chimpanzee
Reemtsma, K. et al. Ann. Surg. 1964, 160, 384-408 - 1970: Liver, Baboon
Bertoye, A. et al. Lyon Chir. 1969, 222, 347-354 - 1971: Liver, Baboon
Pouyet, M. and Berard, P. Lyon Chir. 1971, 67, 288-291 - 1974: Liver, Chimpanzee
Starzl, T. et al. Transplant Proc. 1974, 6, 129-139 - 1977: Heart, Chimpanzee and baboon
Barnard, C. et al. S. Afr. Med. J. 1977, 52, 1035-1038 - 1984: Heart, Baboon
Bailey, L. et al. JAMA 1985, 254, 3321-3329 - 1992: Heart, Pig
Czaplicki, J. J. Heart Lung Transplant. 1992, 11, 393-397 - 1992: Liver, Baboon
Starzl, T. et al. Lancet 1993, 341, 65-71 - 1993: Liver, Pig
Makowka, L. et al. Transplantation 1995, 59, 1654-1659 - 1993: Liver, Baboon
Starzl, T. et al. Immunol. Rev. 1994, 141, 213-244 - 1996: Heart, Pig
Jayaraman, K. Nature 1997, 385, 378 - 2002: α-Gal KO pigs
Lai, L. et al. Science 2002, 295, 1089-1092 - 2009: Attempts to inhibit PERV replication in pigs
Shi, M. et al. Antivir. Res. 2009, 83, 201-204 - 2013: CRISPR/Cas technology used for gene editing of eukaryotic cells
Cong, L. et al. Science 2013, 339, 819-823 - 2015: Using CRISPR/Cas technology to eradicate PERV
Yang, L. et al. Science 2015, 350, 1101-1104 - 2017: Generation of PERV-free pigs
Niu, D. et al. Science 2017, 357, 1303-1307 - 2021: Kidney, Porcine (genetically modified) into brain-dead human subject
Cooper, D.K.C. Xenotransplantation 2021, 28, e12718 - 2021: Kidney, Porcine (genetically modified) into brain-dead human subject
Porrett, P.M. et al. Am. J. Transplant. 2022, 22, 1037-1053 - 2022: Heart, Porcine (genetically modified) into living human recipient
Wang, W. et al. The Innovation 2022, 3, 100223 - 2023: Heart, Porcine (genetically modified) into living human recipient
Griffith, B.P. et al. Nat. Med. 2025, 31, 589-598 - 2024: Kidney, Porcine (genetically modified) into living human recipient
https://hms.harvard.edu/news/first-genetically-edited-pig-kidney-transplanted-human - 2024: Kidney and thymus, Porcine (genetically modified) into living human recipient
https://nyulangone.org/news/first-ever-combined-heart-pump-gene-edited-pig-kidney-transplant-gives-new-hope-patient-terminal-illness - 2025: Clinical trial for United Therapeutics’s UKidney™ set to begin
NCT06878560
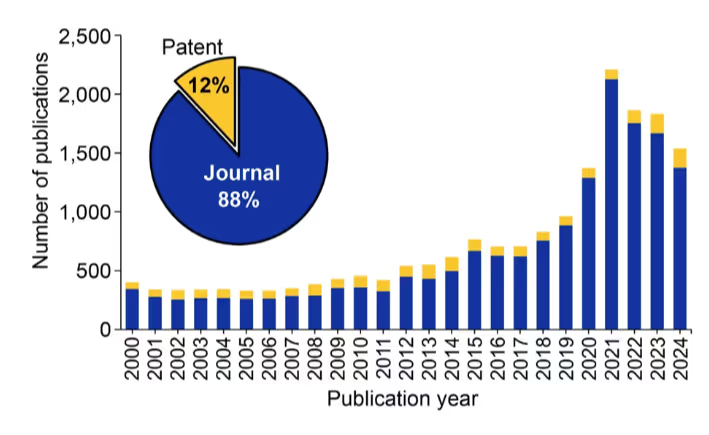
The development of CRISPR/Cas technology has ushered in new advancements and resulted in clinical experimentation with a spate of xenotransplantations being reported in 2022-2024. We examined the CAS Content CollectionTM, the largest human-curated repository of scientific information, and found consistent growth in publications relating to xenotransplantation (see Figure 1).
Publications peaked in 2021, right before the latest genetically modified transplants began. The dominance of journal publications and relatively small number of patents show that this technology is still far from widespread commercialization, yet research remains steady.
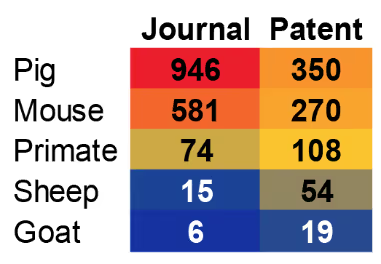
The animal species most commonly studied and utilized in xenotransplantation are shown in Figure 2. Pigs are by far the animal of choice for these studies, and as we’ll see, pig organs have experienced the greatest advances in genetic engineering and transplantation.
There are several reasons why pigs are the animal of choice for xenotransplantation, including:
- Anatomical similarities with humans (organ size).
- Physiological similarities with humans.
- Relatively lower cost due to ease of breeding and rapid reproduction.
- Relative ease and highly compatible with genetic modifications.
- Lower risk of zoonotic diseases.
Barriers to xenotransplantation
Xenotransplanted organs can face several types of rejection: hyperacute (occurring almost immediately, in a matter of minutes to hours), acute (occurring weeks to months after transplantation), and chronic rejection (occurring months to years after transplantation).
Organ rejection is mediated by many factors including innate and adaptive immunity (see Table 1). Innate immunity is controlled by natural antibodies that bind to and recognize porcine glycoproteins (often referred to as xenoantigens). Some of the identified xenoantigens include galactose-α-1,3-galactose (α-Gal), N-glycolylneuraminic acid (NeuGc), and SDa.
Since the 1990s, considerable research efforts have been directed to create pigs with the genes for these proteins knocked out to avoid hyperacute rejection. Knocking out α-Gal in pigs was considered an important milestone in the advancement of xenotransplantation. In recent years, triple knockout (TKO) pigs are becoming more prevalent.
Table 1: Major differences in innate vs. adaptive immune systems.
Other components of the innate immune system that play a role in organ rejection include macrophages and natural killer (NK) cells. The former plays a role in rejection by mediating inflammation (release of proinflammatory cytokines, reactive oxygen species, etc.) and phagocytosing cells. CD47, a transmembrane protein expressed on immune cells such as T- and B-cells, interacts with signal regulatory protein α (SIRPα), which are inhibitory receptors expressed on macrophages and NK cells, to prevent phagocytosis. Porcine CD47 does not appear to interact with SIRPα similar to human CD47 and therefore cannot prevent phagocytosis. In recent years, several studies have explored the feasibility of expressing human CD47 in pig organs to reduce organ rejection. However, CD47 has many other roles besides its interaction with SIRPα, and the expression of human CD47 in porcine organs might have unintended consequences.
Similar to macrophages, NK cells can also lead to organ rejection either directly or indirectly. Like macrophages, NK cells also express inhibitory and activating receptors on their surface that interact with binding partners and modulate their activity. Binding HLA class I molecules to killer cell immunoglobulin-like receptors (KIR), a type of inhibitory receptor on NK cells, is thought to prevent the activation of NK cells.
Unlike innate immunity, adaptive immunity occurs in response to exposure to foreign substances using B- and T-cells. B-cell receptors are membrane-bound antibodies expressed on the surface of B-cells that recognize antigens and result in B-cell activation and an immune response. A 2012 article reported prolongation of porcine cardiac xenograft survival in baboons by 2.5-fold by incorporating antibodies against CD20, a protein expressed on the surface of B-cells, in the immunosuppressive regimen. Others have also reported using antibodies against CD40, cell-surface receptors found on B-cells, macrophages and other immune cells, in combination with other immunosuppressive agents to prolong survival of porcine islet xenografts in non-human primates.
T-cells are another type of white blood cell similar to B-cells, but T-cells undergo further development and maturation in the thymus gland. The activation of T-cells by the interaction of T-cell receptors (TCR) with major histocompatibility cells (MHC) on antigen-presenting cells in the presence of co-stimulatory molecules triggers a complex cascade of intracellular signaling and generation of T-cell subtypes.
Evidence suggests that in pig-to-human xenotransplantation, porcine MHC molecules called swine leucocyte antigen (SLA) bind to TCR on human T-cells causing activation and resulting in an immune response. Efforts are continuing to create donor pigs with reduced SLA expression to lower T-cell activation.
Besides organ rejection mediated by immune response, inflammation and coagulative dysfunction are major hurdles in successful xenotransplantation. The liver plays a key role in the synthesis of many clotting factors. While similarities exist between human and porcine coagulation systems, fundamental differences also contribute to incompatibilities in clotting and bleeding complications.
Inflammation is considered a normal physiological response upon exposure to detrimental stimuli that is attenuated once the stimuli is addressed. There is mounting evidence, however, that systemic inflammation may occur following xenotransplantation and that this systemic inflammatory response contributes to issues with coagulation and immune response, all of which contribute to xenograft/xenotransplant failure.
Considering the complex interconnections between the inflammatory and coagulation systems, controlling systemic inflammation likely aids coagulation dysfunction reduction and therefore helps prolong survival following xenotransplantation, either by using anti-inflammatory drugs or by expressing proteins such as hemeoxygenase-1 (HO-1) and others having anti-inflammatory effects in donor pigs.
Lastly, viruses such as porcine endogenous retroviruses (PERVs) and porcine cytomegalovirus (PCM) have been reported to be transmitted across species, including humans, in xenotransplantation trials and experiments. One potential contributing factor to a recent xenotransplantation failure may have been a reaction to latent PCM.
These challenges demonstrate how complex xenotransplantation is and why genetic editing shows so much promise in this field.
Why CRISPR could be the key to successful xenotransplantation
CRISPR (clustered regularly interspaced short palindromic repeats), a part of the bacterial defense mechanism, is a revolutionizing gene editing technology with the first CRISPR/Cas9-based therapy, Casgevy, gaining U.S. FDA approval in 2023.
The main avenues for which CRISPR/Cas technology is used in the context of xenotransplantation include:
- Knocking out porcine genes that encode proteins acting as antigens and whose absence help overcome hyperacute rejection and overall decrease rejection of the xenotransplanted organ.
- Increasing the immuno-compatibility of transplanted donor organs by expression of human transgenes.
- Lowering the risk of zoonotic diseases such as PERV by eliminating genes associated with it.
- Controlling the size of donor organs.
There are two common routes through which genetically modified pigs can be created using CRISPR/Cas technology. The first incorporates mRNA for Cas9 nuclease and guide RNA (gRNA) into porcine primary cells using transfection, followed by successive rounds of cell culture and somatic cell nuclear transfer, a technique utilized to transfer the nucleus of a somatic cell to egg cells, and finally breeding to give rise to genetically modified pigs. The other route involves microinjecting porcine zygotes with Cas9 nuclease and gRNA expression vectors or RNA, followed by breeding to produce genetically modified pigs.
Table 2 lists genes that have been modified using CRISPR/Cas technology in xenotransplantation research. Most genetically modified pigs used in xenotransplantation research tend to have more than one genetic modification, e.g., double, triple, and quadruple knocked-out (KO) pigs.
Porcine genes (knocked out) and related proteins
- GGTA1: Glycoprotein α-galactosyltransferase 1
- CMAH: N-glycolylneuraminic acid
- β4GALNT2: β-1,4-acetyl-galactosaminyltransferase
- A3GALT2: α-1,3-galactosyltransferase 2
- PERV: Porcine endogenous retroviruses
- GHR: Growth hormone receptor
- β2M: β2-microglobulin,
- CIITA: class II majorhistocompatibility complex transactivator
- SLA: Swine leucocyte antigen
Human genes (knocked in) and related proteins
- CD39: Ectonucleoside triphosphate, diphosphohydrolase 1
- CD46: Membrane cofactor protein
- CD47: Integrin associated protein
- CD55: Decay-accelerating factor
- CD59: MAC-inhibitory protein
- β2M: B-2-microglobulin
- HLA-E: Major histocompatibility complex, class I, E
- THBD: Thrombomodulin
- TFPI: Tissue factor pathway inhibitor
- HO1: Heme oxygenase-1
- EPCR: Endothelial protein C receptor
Table 2: List of genes that have been modified for xenotransplantation using CRISPR/Cas technology.
Reports suggest that porcine hearts continue to grow after transplantation into non-human primates, leading to failure. This also requires the administration of drugs that can reduce this growth such as mTOR inhibitor, temsirolimus, and others. One way to overcome this issue of continued growth is by knocking out the growth hormone receptor in pigs, resulting in overall smaller pigs and organs. For the time being, this issue is confined to pre-clinical non-human primate models for xenotransplantation though controlling the size of the xeno-organs may become important in case of pediatric xenotransplantations.
As noted, CRISPR/Cas technology has resulted in several key xenotransplantation breakthroughs in recent years. These include:
- Pig heart transplant into a living human recipient in 2022. The porcine heart was extensively genetically modified to boost the chances of survival. Genetic modifications included knocking out genes of three xenoantigens (galactose-α-1,3-galactose, Sda blood group antigen, and N-glycolylneuraminic acid), knocking out growth hormone receptor genes to control the size of the organ, and introducing a total of six human genes in a bid to humanize the porcine heart and improve compatibility (CD46, decay-accelerating factor, thrombomodulin, endothelial cell protein C receptor, CD47, and heme oxygenase 1). While there was no obvious evidence of xenograft rejection, the patient passed away two months after the xenotransplantation occurred. While unfortunate, this case is nonetheless a remarkable achievement/milestone in the field.
- Pig heart transplant into a living human recipient in 2023. Conducted by the same team of doctors and researchers as the xenotransplantation in 2022, a similarly genetically modified porcine heart was used. As in 2022, the patient passed away six weeks after surgery. In both instances, a marked increase in the weight of the heart accompanied by “myocardial thickening” was observed during the autopsy.
- Pig kidney transplant into a living human recipient in 2024. Like the porcine heart xenotransplantations described above, the porcine kidney was also genetically modified, reportedly consisting of 69 genomic edits accomplished using CRISPR/Cas technology. The patient passed away nearly two months after the surgery.
- Pig kidney and thymus transplant into a living human recipient in 2024. This procedure was unique in that the transplanted organ, dubbed UThymoKidney™ (United Therapeutics Corporation). It consisted of a porcine kidney combined with thymus tissue from the same pig implanted into it to decrease the likelihood of rejection by preventing the donor’s immune system from recognizing the transplanted organ as a foreign object. Unlike the other cases, the xenotransplant was removed from the donor’s body a month and a half after the surgery due to organ failure, requiring resumption of dialysis with the patient eventually passing away after a few weeks.
Despite the continual challenges of post-surgical survival, clinical trials are proceeding. For example, in 2025, United Therapeutics announced the U.S. FDA approval of its Investigational New Drug application (IND) to study the UKidney™ in a clinical trial. The Phase1/2 clinical trial is set to start this summer, and it intends to study the effects of xenotransplantation of 10 genetically engineered kidneys (UKidney™), resulting from 10 gene modifications — a mix of knocking out four pig genes and adding six human genes in patients with end-stage renal disease.
Beyond organ transplants
Xenotransplantation procedures may also address conditions other than organ failure. Thanks to gene editing techniques like CRISPR, animal cells and tissues may be modified to treat additional human diseases.
Type 1 diabetes:
Transplantation of islets from deceased human donors to patients with type 1 diabetes has been an ongoing area of interest, but demand for islets outstrips supply. In recent years, the transplantation of genetically modified porcine islets with knocked-out xenoantigens and humanization by expression of human proteins in non-human primates have shown promising results. However, several fundamental challenges remain, including initial inflammatory and immune reaction to the transplant (IBMIR) as well as differences in islet physiology and insulin secretion.
Polycystic ovarian syndrome (PCOS)
Recently, scientists from the Nanjing Medical University in China tried xenotransplantation of brown adipose tissue from rats into mice to treat PCOS. Brown adipose tissue, commonly abbreviated as BAT, is a type of fat that is involved in using lipids and glucose for thermogenesis and body temperature regulation. The xenotransplantation of rat BAT into mice allowed for partial correction of PCOS and improved the overall performance of ovaries in the mice. These early experimental studies appear promising and are a ray of hope to individuals suffering from PCOS, nearly six million in the U.S. alone.
Cancer
The application of xenotransplantation in cancer involves developing patient-derived xenografts, which are models created by implanting cancerous tissues and cells from patients into animals such as mice and rats. These models are invaluable to understanding the pathophysiology of the disease, testing and evaluating more efficient drugs, and designing personalized medicine.
Challenges and opportunities with xenotransplantation
For all the improvements in xenotransplantation, significant hurdles remain to widespread clinical usage. The complexity of immune compatibility means that reliance on genetic engineering alone may not be able to prevent organ rejection. There are also technical and ethical challenges to address, namely potential infections and differences in organ sizes, as well as questions about consent and animal welfare.
The medical community also needs to understand any long-term implications of xenotransplantation and have a consistent, robust regulatory framework to guide future efforts. Because of these challenges, xenotransplantation is still considered an experimental line of treatment with numerous barriers to overcome.
However, the advancements in genetic engineering that have led to tangible improvements provide an undeniable impetus to this field. With continued breakthroughs in gene editing and immunological understanding, xenotransplantation could provide hope for thousands or even millions of patients in need of organs and tissues.