摘要
极端微生物是指在极端环境中繁衍生息的生物体,不断挑战着我们对生命存续与适应机制的认知边界。这些生物体展现出惊人的多样性及独特的生化适应机制,例如极端酶与抗逆性细胞结构便是典型例证。极端微生物已经进化出一套生化途径,能够生成具有极高稳定性和生物活性的生物活性化合物。这些极端微生物代谢产物(包括抗菌肽、极端酶、抗癌制剂、抗氧化剂等)在制药、生物技术、生物修复、农业、生物燃料生产等众多行业蕴含着巨大的应用潜力。基因组学、宏基因组学与合成生物学领域的最新进展,加速了从这些顽强生物中发现新型生物活性化合物的进程,为应对抗生素耐药性、工业催化和环境可持续性等全球性挑战提供了创新解决方案。
简介:何为极端微生物?
地球生命展现出惊人的适应能力,甚至能在最恶劣的环境中繁衍生息。被称为极端微生物的生命体,在曾被认为无法生存的极端环境中依然蓬勃繁衍,包括灼热的深海热泉、强酸或强碱湖泊、高盐度水域以及南极的冰冻荒原。对这些顽强微生物的研究彻底改变了我们对生命边界的认知,为进化生物学、生物技术乃至天体生物学提供了关键启示。
极端微生物根据其栖息的极端环境类型进行分类。它们包括嗜热微生物(高温)、嗜冷微生物(低温)、嗜酸微生物与嗜碱微生物(极端 pH 值)、嗜盐微生物(高盐度)、嗜压微生物(高压)以及嗜干微生物(极端干燥环境)等。它们的生存策略通常包括被称为“极端酶”的特化酶、独特的生物膜结构、DNA 修复机制以及能够抵御极端理化压力的代谢途径。
极端微生物的发现已经产生了深远的影响。在生物技术领域,从嗜热微生物水生栖热菌中提取的极端酶 Taq 聚合酶彻底革新了 PCR 技术,而来自嗜盐微生物和嗜碱微生物的酶类则被广泛应用于洗涤剂、食品加工和废物处理中。此外,极端微生物在生物修复中发挥着关键作用,能在常规微生物无法生存的极端环境中降解污染物。
从天体生物学的视角来看,极端微生物可作为潜在外星生命的类比研究对象。永久冻土层中的产甲烷古菌、深海热泉的硫代谢古菌以及耐辐射细菌,为揭示生命在火星、木卫二或土卫二这类天体上的存续机制提供了重要线索。因此,对极端微生物的研究架起了地球生物学与地外生命探索之间的桥梁。
然而,尽管极端微生物意义重大,其生物学的诸多方面仍待探索。它们在极端气压下如何演化?在地球最偏远的生态角落中,潜藏着哪些尚未被发现的物种?能否进一步利用其独特的生物化学特性来推动可持续技术的发展?对不可培养物种的宏基因组挖掘、在模式宿主中的异源表达,以及利用纳米技术辅助的递送系统,这些创新性解决方案正在帮助解决这些关键问题。
在本报告中,我们探索了全球最大人工标引科学信息库 CAS 内容合集™ 中的数据,以深入解读极端微生物领域的研究进展。从海洋深处到核禁区,这些微小生物正推动着药物研发、环境修复等诸多科学挑战领域的创新突破。
极端微生物的种类及其生存方式
“极端微生物”这一术语由 MacElroy 于 1974 年提出,用以指代能在极端环境中生存的生物体。尽管包含部分原生动物、藻类和真菌物种,但绝大多数极端微生物属于原核生物,归类于古菌域和细菌域,它们通过独特的代谢与生理适应机制得以在这些严酷环境中生存。
如前所述,极端微生物生存于难以置信的高温或低温环境、高盐度或极端 pH 值环境,以及诸如深海海沟等极端高压环境中。此外,还存在具有辐射抗性的极端微生物,它们能够在沙漠和核辐射区域存活。例如,切尔诺贝利枝孢菌,一种来自切尔诺贝利隔离区的真菌,表现出多样的生存策略,如 DNA 修复和黑色素生成。如表 1 所示,极端微生物的多样性遍及诸多恶劣环境:
| 类型 | 极端条件 | 代表性生物 | 自然栖息地 | 关键适应机制 | 应用 |
|---|---|---|---|---|---|
| 嗜热微生物 | 高温(45–80°C) | Thermus aquaticus、Pyrococcus furiosus | 温泉、地热喷口 | 耐热酶,修饰的膜脂质 | PCR(Taq 聚合酶),工业催化 |
| 超嗜热微生物 | 极高温度(>80°C) | Methanopyrus kandleri (122°C) | 深海热液黑烟囱 | 反向 DNA 旋转酶、分子伴侣蛋白 | 极端条件生物反应器工艺 |
| 嗜冷微生物(喜冷微生物) | 低温(<15°C) | Psychrobacter, Polaromonas | 极地冰盖、深海区域 | 抗冻蛋白、柔性膜 | 低温洗涤剂、食品保鲜 |
| 嗜酸菌 | 低 pH 值 (<3) | Picrophilus torridus (pH -0.06) | 酸性矿山排水、火山池 | 质子泵、耐酸细胞壁 | 金属生物浸出、酸性矿山排水清理 |
| 嗜碱菌 | 高 pH 值(>9) | Natronomonas pharaonis (pH 11) | 碱湖、碳酸盐土壤 | 钠氢反向转运蛋白、特化转运蛋白 | 洗涤剂用酶、纺织加工 |
| 嗜盐菌 | 高盐度(2–5M NaCl) | 盐沼盐杆菌 | 盐滩、高盐湖 | 积累相容性溶质(如氯化钾)、利用细菌视紫红质产能 | 生物塑料、太阳能盐生产 |
| Piezophiles(Barophiles) | 高压环境(>400 个大气压) | 深海假单胞菌(马里亚纳海沟) | 超深渊海沟、深层地下环境 | 压力稳定蛋白、不饱和脂肪酸 | 深海生物技术、高压废物降解 |
| 耐辐射微生物 | 高电离辐射 | 耐辐射球菌 | 核废料场,沙漠 | 高效的DNA修复,Mn²⁺-抗氧化复合物 | 核废料净化、辐射防护药物 |
| 嗜干微生物 | 极端干燥 | Chroococcidiopsis(沙漠结皮) | 沙漠环境、干燥食品 | 海藻糖积累,DNA/蛋白质保护 | 抗旱作物、疫苗稳定性保护 |
| 寡营养微生物 | 营养贫瘠环境 | Pelagibacter ubique(海洋) | 开阔海域、深层地下水 | 超高效代谢、小基因组 | 废水处理、低营养生物加工 |
| 耐金属微生物 | 高浓度重金属环境 | 嗜酸铁原体(铜/砷耐受菌) | 矿山尾矿、工业废料 | 金属外排泵、金属硫蛋白 | 有毒金属的生物修复 |
| 厌氧 | 无氧环境 | 产甲烷古菌、梭菌属 | 肠道微生物组、深层沉积物 | 替代电子受体(如 SO₄²⁻、CO₂) | 沼气生产、肠道微生物组研究 |
| 岩内微生物 | 岩石内部 | Chroococcidiopsis(南极岩石) | 南极干谷、地下环境 | 抗紫外线色素、缓慢代谢 | 地球化改造模型、生物特征探测 |
| 嗜二氧化碳生物 | 高浓度二氧化碳环境 | 弯曲杆菌 | 哺乳动物腔、废水 | 碳固定嗜二氧化碳乳酸发酵 | 二氧化碳生物固定、病原菌培养 |
| 多重嗜极生物 | 多重极端环境微生物(如嗜热嗜酸微生物) | Sulfolobus acidocaldarius (75°C + pH 3) | 火山热酸泉(75°C + pH 3) | 上述适应的组合 | 太空模拟研究、多用途工业酶 |
极端微生物展现出非凡的生存机制,使其能够在地球最恶劣的环境中繁衍生息。这些微生物通过进化形成了精妙的生化、结构和基因组适应机制,以耐受极端温度、pH 波动、高盐度、干燥及辐射等恶劣条件。它们的一些关键生存机制包括:
- DNA 和蛋白质保护:嗜极生物通过多种方法保护其遗传物质和蛋白质免受变性和损伤:
- 热休克蛋白 (HSP):诸如激烈火球菌中的 HSP70 等分子伴侣,能在高温环境下有效防止蛋白质错误折叠。
- 反向旋转酶:这种存在于超嗜热微生物中的酶能引入正超螺旋,使 DNA 在超过 100°C 的高温下保持稳定。
- 抗辐射 DNA 修复:耐辐射奇球菌利用同源重组与核苷酸切除修复机制,在遭受极端辐射后重组破碎的染色体。
- 膜与细胞壁适应性:极端微生物通过以下方式调整其膜结构以维持流动性和完整性:
- 醚键脂质:诸如硫化叶菌等古菌利用四醚脂质来抵抗极端高温与酸性环境。
- 单层膜结构:部分超嗜热微生物通过形成脂质单层膜来增强热稳定性。
- 耐酸细胞壁:嗜酸菌属微生物在 pH 值为 0 的条件下,仍能通过不可渗透膜维持质子梯度。
- 嗜盐微生物的渗透调节:嗜盐微生物通过以下方式在高盐环境中存活:
- 相容性溶质:盐沼盐杆菌通过积累钾离子和有机渗透剂(如甜菜碱)来平衡渗透压。
- 盐内策略:部分嗜盐微生物通过积累高浓度细胞内盐分,并依赖仅在近饱和盐度下起作用的特化嗜盐酶来维持生命活动。
- 代谢灵活性:许多极端微生物能利用非常规能源:
- 无机化能营养:嗜酸氧化亚铁硫杆菌在酸性矿山环境中通过氧化铁和硫获取能量。
- 厌氧产甲烷: 像 Methanopyrus kandleri 这样的产甲烷菌在热液喷口中产生甲烷。
- 辐射合成代谢:部分细菌能利用放射性衰变产生的水分子辐射分解作用,在深层地下环境中获取能量。
- 隐生与休眠状态:部分极端微生物在胁迫环境下会进入生命活动暂停状态:
- 耐旱休眠:缓步动物和某些细菌(如色球藻属)通过产生海藻糖来保护细胞结构,从而能够在完全脱水的情况下生存。
- 孢子形成:芽孢杆菌与梭菌属物种能生成耐热、抗辐射且抵御化学物质的内生孢子。
新型极端微生物的最新发现
微生物生态学与基因组学的最新进展显著拓展了我们对极端微生物多样性的认知,揭示了极端环境中新的分类群及此前未被探索的适应策略。高通量测序与单细胞基因组学技术揭示了栖息在热液喷口、超酸性湖泊、极地冰盖及深层地下生物圈中古菌和细菌的神秘谱系。对极端微生物群落的宏基因组学研究发现了一些独特的代谢途径,例如深海嗜热微生物中新型的化能无机自养机制,以及嗜盐古菌中的混合型光合作用系统。
在热泉中发现的阿斯加德古菌具有重要意义,它为真核生物进化提供了新见解;而从核废料场分离出的具有超强 DNA 修复能力的耐辐射细菌,同样令人瞩目。科学家还从秘鲁盐滩分离的耐盐枯草芽孢杆菌 CH11 菌株中,发现了一种新型 II 类L-天冬酰胺酶。开发具有更高稳定性与效能的 L-天冬酰胺酶变体,是该酶在食品工业和癌症治疗领域得以广泛应用的关键目标。
近期研究显示,洞穴中存在着独特的原核生物群落,其特化类群已适应了局部的能量与营养条件。在罗马尼亚莫维尔洞穴的硫化物水域中,科学家发现了新物种伪康顿虫,该洞穴拥有独特的化能自养生态系统,生命依靠硫化氢氧化而非光合作用维持。此外,极端微生物(尤其是嗜盐微生物)已在废弃的铜矿环境中演化生存。
细菌型极端微生物,尤其是候选门级辐射类群 (CPR) 细菌,是一类极具研究价值的微生物群体,特别是那些最新发现于酸性矿山排水环境中的菌种。其特征表现为超小细胞体积、简化的基因组结构,以及对宿主生物相互作用的依赖性。
耐辐射奇球菌及其近缘物种(如奇球菌科的其他成员)以其卓越的辐射抗性著称,尤其能在核反应堆内部或周边等高强度电离辐射环境中存活。它们已演化出高效修复系统,能够修复由辐射及干旱胁迫等因素引发的 DNA 损伤。
此外,非培养技术已在极端环境中揭示了“微生物暗物质”,这表明其中蕴藏着尚未开发的系统发育和功能多样性宝库。这些发现不仅重新划定了地球生命的生存边界,更为天体生物学、生物技术及气候变化应对策略等领域带来了深远影响。
然而,在培养这些微生物并将基因组学认知转化为功能性理解方面,我们仍面临挑战。未来研究应将多组学方法与先进培养技术相结合,以深入探索这些新发现极端微生物的生态功能与生物技术潜力。
来自极端微生物的生物活性化合物
极端微生物能产生独特的生物活性化合物,在医药、工业和生物技术领域具有应用潜力(参见表 2)。这些次级代谢产物(包括酶、抗菌剂和抗氧化剂)在严苛条件下仍能表现出卓越的稳定性与功能性。极端微生物勘探领域的最新进展揭示了一批具有抗癌、抗炎及抗菌活性的新型治疗性化合物。此外,源于极端微生物的酶类(极端酶)在极端 pH、温度及盐度条件下仍能保持强稳定性,因此在工业流程中极具应用价值。这些化合物为应对耐药性、工业催化及环境可持续性等挑战提供了创新性解决方案。
最新研究表明,超过 40% 的微生物活性化合物尚未被发现,而极端微生物正是一个尚未被充分开发的巨大资源宝库。CRISPR-Cas 系统(来源于 嗜热链球菌)和 Taq 聚合酶(来源于水生热杆菌)是最成功的极端微生物衍生生物技术工具之一。嗜盐微生物、嗜热微生物和嗜酸微生物能产生具有工业应用价值的抗菌素、抗癌剂及可生物降解聚合物。
| 极端微生物类型 | 关键生物活性化合物 |
|---|---|
| 嗜热微生物 | 耐热酶类(DNA 聚合酶、蛋白酶)、抗菌肽(硫化叶菌素) |
| 嗜冷菌 | 抗冻蛋白、冷活性酶(脂肪酶、蛋白酶) |
| 嗜盐菌 | 菌红素(抗氧化剂)、盐霉菌素(抗菌肽) |
| 嗜酸菌/嗜碱菌 | 耐酸纤维素酶、嗜碱性蛋白酶 |
| Piezophiles(Barophiles) | 耐压酶类、生物活性胞外多糖 |
| 抗辐射 | DNA 修复酶、辐射防护化合物 |
各种组学技术和生物信息学的进展揭示了许多先前未知的次级代谢产物、肽类和极端酶,它们在极端条件下表现出显著的生物活性。这些极端酶和次级代谢物相比传统药物具有优势,包括热稳定性(有利于存储和递送)、新颖的结构(绕过现有的抗药性机制)和高特异性(减少脱靶效应)。
突破性发现包括:
- 源自深海嗜热微生物的超耐热抗菌肽,能通过新型成孔机制破坏细菌细胞膜。
- 源自奇球菌属的耐辐射色素,通过独特的自由基清除途径展现出强效抗氧化活性。
- 源自硫化叶菌的酸稳定性抗生素,通过修饰型硫醚桥结构靶向耐药病原体,具有抑制细胞壁合成与破坏膜电位的双重作用机制。
这些在极端条件下赋予生物活性的结构适应性尤为显著,例如嗜盐菌细菌素中的 D-氨基酸嵌入、嗜压化合物中的耐压折叠结构,以及嗜冷代谢物中的抗冻修饰机制。这些化合物的分子靶点同样值得关注:包括与微生物膜的相互作用(如嗜热脂肽锚定脂质 II)、对必需酶的抑制(如深海盐孢菌素对蛋白酶体的抑制),以及对核酸代谢的干扰(如耐辐射细菌代谢物对 DNA 的嵌入作用)。
创新性的发现方法已经彻底改变了极端微生物的生物资源开发,包括:通过多组学指导的基因组挖掘、在合成生物学平台上的异源表达,以及在模拟极端环境下进行的高通量活性筛选。这些创新方法已成功转化为医学应用,例如针对多重耐药性 ESKAPE 病原体的新一代抗生素。在农业领域,基于极端代谢物的生物刺激素已被成功研发,而适用于绿色化学的溶剂稳定酶正推动工业生物技术革新。
研究人员正通过运用计算模型与 CRISPR 技术驱动的通路工程等解决方案,着力应对化合物规模化制备及构效关系优化等挑战。对极端微生物活性物质的发现及其作用机理的系统性整合,为未来研究提供了清晰路线图,也为应对全球健康与环境挑战的创新解决方案奠定了坚实基础。
表 3 列出了目前已开发的极端微生物来源药物/活性制剂及其应用领域。其中包括美国 FDA 已批准的 L-天冬酰胺酶和 Taq 聚合酶;颇具前景的临床前候选药物(如对抗抗生素耐药性的盐霉菌素);以及可用于癌症治疗的菌红素。
| 极端微生物来源 | 生物活性剂 | 疾病靶点/应用领域 | 作用机制 |
|---|---|---|---|
| 抗癌制剂 | |||
| Thermus thermophilus(嗜热菌) | L-天冬酰胺酶 | 急性淋巴细胞白血病(ALL) | 消耗天冬酰胺以饿死癌细胞 |
| 盐沼盐杆菌(嗜盐菌) | Bacterioruberin | 乳腺癌/结肠癌 | 抗氧化剂,诱导细胞凋亡 |
| Picrophilus torridus(嗜酸菌) | 耐酸蛋白酶 | 胰腺癌(酸性肿瘤) | 在低 pH 值肿瘤微环境中激活前体药物 |
| 耐辐射奇球菌(抗辐射微生物) | 锰复合物 | 辐射防护(癌症治疗) | 清除 ROS,保护健康细胞 |
| 抗菌与抗真菌制剂 | |||
| Haloferax mediterranei(嗜盐菌) | 盐霉菌素 | MRSA 和假单胞菌感染 | 破坏细菌细胞膜 |
| Sulfolobus solfataricus(嗜热酸菌) | 硫化叶菌素 | 白色念珠菌(真菌感染) | 结合麦角甾醇(真菌膜) |
| 科尔韦氏菌(嗜冷菌) | 抗冻蛋白 | 生物膜预防(植入器械) | 抑制细菌黏附 |
| 神经退行性疾病疗法 | |||
| Pyrococcus furiosus (超嗜热菌) | 伴侣蛋白 | 阿尔茨海默病/帕金森病 | 防止蛋白质错误折叠 |
| 耐辐射奇球菌(抗辐射细菌) | 超氧化物歧化酶 | 帕金森病 | 减少帕金森病中的氧化应激 |
| 抗病毒与基因编辑 | |||
| 嗜热链球菌(嗜热菌) | CRISPR-Cas9 | HIV基因治疗 | 利用基因编辑技术切除病毒DNA |
| Thermus aquaticus(嗜热菌) | Taq聚合酶 | 病毒诊断(HIV、HPV、COVID-19) | 病毒 DNA 的 PCR 扩增 |
| 抗炎和免疫调节剂 | |||
| Alteromonas macleodii(深海) | 胞外多糖 | 类风湿性关节炎 | 抑制TNF-α(抗炎) |
| 抗氧化剂和紫外线防护剂 | |||
| 嗜盐菌 | Bacterioruberin | 营养保健品与抗衰化妆品 | 抗氧化活性强度是 β-胡萝卜素的四倍 |
| 蓝细菌、微藻、激烈火球菌(嗜热菌) | 类菌孢素氨基酸 (MAA) | 护肤产品中的天然防晒成分 | 在 UV-A 和 UV-B 区域具有很强的吸收能力 |
| 工业极端酶 | |||
| Thermus aquaticus | Taq聚合酶 | 分子生物学中的 PCR 扩增 | 防止酶在加热过程中变性 |
| 嗜酸菌 | 漆酶 | 纺织品染料的生物降解与生物修复 | 通过催化氧化反应促进纺织品染料的生物降解与解毒过程 |
| 嗜冷菌 | 冷活性蛋白酶 | 环保洗涤剂与食品加工 | 破坏活性位点的稳定性,增强其柔性,使酶在低温环境下仍能保持活性 |
尽管潜力巨大,极端微生物的培养与提取技术仍是当前的主要瓶颈。目前许多极端微生物仍难以实现人工培养,且尚需开展更多临床试验。未来研究应结合宏基因组学与合成生物学技术,以高效开发利用这些生物活性分子。
尽管如此,极端微生物所承受的独特进化压力,仍产生了具有无可比拟特性和新颖作用机制的生物活性化合物。随着探测技术的进步,这些生物体将在应对全球健康与环境挑战中发挥至关重要的作用。对其生物活性化合物的系统性研究,不仅拓展了我们的药物资源库,更为理解生命的适应性提供了基础性认知。
研究布局和新突破
我们对 CAS 内容合集的分析表明,过去 25 年间与极端微生物相关的文献数量已增长至原先的三倍。专利数量相对较少,仅占全部文献的约 5%,但自 2000 年以来,每年的专利数量已增加了四倍(见图 1)。
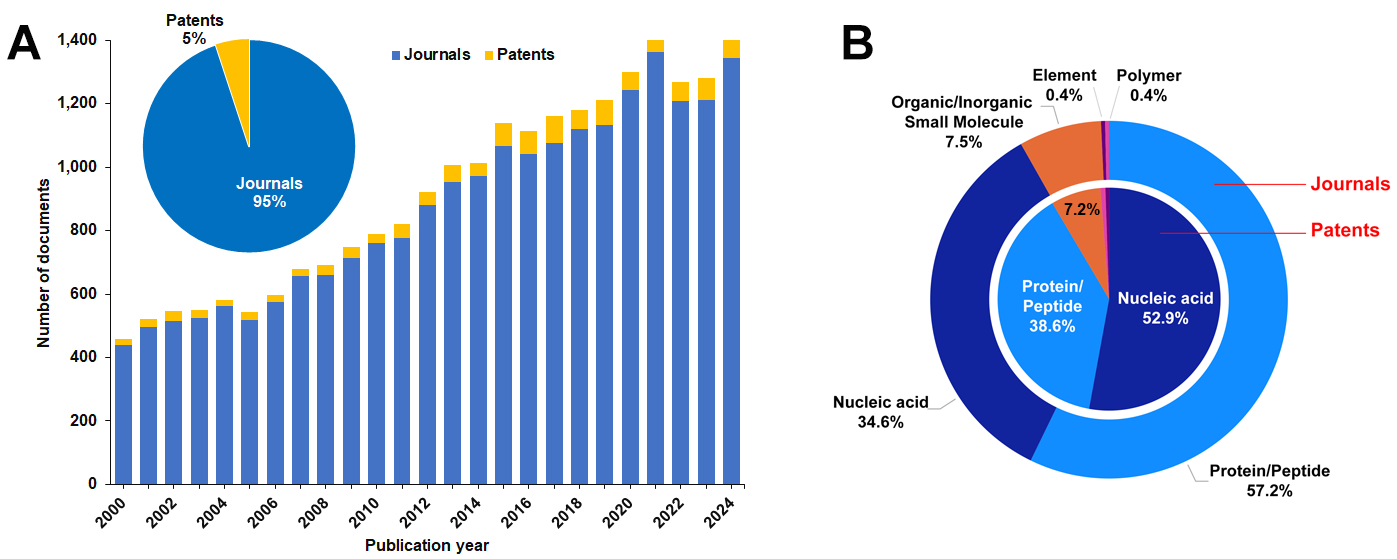
我们的分析表明,蛋白质/肽类与核酸和极端微生物的关联最为密切。在专利文献中,核酸相关主题占比不足 50%;而在期刊文献中,蛋白质/肽类研究占据主导地位,约占出版物总量的 60%。小分子相关研究约占文献总量的 8%(参见图 1B)。
我们还研究了相关文献中嗜极生物类型的分布情况(查看图 2)。

从这些分析中,我们发现了一些重要启示:
与极端微生物相关的文献中,数量最庞大的研究对象当属寡营养微生物,这类生物能在营养匮乏的环境中蓬勃繁衍
寡营养环境(例如开阔海洋、深层地下及沙漠)覆盖了地球的大部分区域。因此,寡营养微生物驱动着多项关键生态过程:在贫营养海域实现碳封存、在贫瘠土壤中完成氮磷循环,并在极端生态系统中维持微生物多样性。它们凭借极少的资源便能旺盛生长的能力,使其在生物修复、生物能源(例如只需少量底物的微生物燃料电池)以及适应极端贫瘠环境的新型酶类和生物活性化合物开发中极具价值。随着气候变化导致贫营养环境不断扩张,理解寡营养微生物的生存策略有助于预测微生物对生态系统变化的响应,以及变暖海洋中碳储存格局的演变。
此外,寡营养微生物能在类似于火星、木卫二或土卫二等营养匮乏星球的严苛环境中存活。对它们的研究有助于识别潜在的地外生命、为太空任务制定生命探测策略,并理解这些环境中的生命存续机制。早期地球养分有限,使得寡营养微生物可能成为研究远古生命的理想参照模型。对它们的研究为揭示原始微生物的适应机制,以及低营养条件下代谢途径的起源提供了关键线索。
过去五年间,出版物数量增长最显著的极端微生物类别依次为:多极端微生物(约 51%)、嗜金属微生物(约 47%)以及耐辐射微生物(约 36%)
- 多极端微生物是能在多种极端条件下同时繁衍生息的生物体,近年来科学关注度激增,因为它们不仅突破了生命的生存极限,更为生物学、天体生物学和生物技术领域提供了前所未有的研究视角。多极端微生物能产生在极端工业条件下仍保持稳定的多功能酶和生物分子。随着地球面临气温上升、海洋酸化和荒漠化等挑战,多极端微生物的研究有助于预测生态系统崩溃时微生物的适应力、永冻层融化过程中的碳循环机制,以及环境剧变下的生物演化路径。
- 嗜金属微生物是一类能在高浓度有毒金属(如砷、汞、镉、铀)环境中蓬勃生长的微生物。它们在修复污染场址方面发挥着关键作用,包括矿山尾矿、工业废料、核废料场所以及汞砷污染水域的治理。它们固定或转化有毒金属的能力,为化学治理提供了一种低成本、生态友好的替代方案。嗜金属微生物在生物采矿中也发挥着关键作用,它们能从低品位矿石中提取铜、金和稀土元素等有价值的金属,从而减少采矿过程中对氰化物等有毒化学品的依赖。嗜金属微生物通过产生独特的生物分子来抵御金属毒性,包括金属结合肽和铁载体,这些物质有望成为新型抗生素的来源。它们体内含有能解除重金属毒性的酶类,这些酶可用于化疗药物研发;同时它们还能产生耐辐射化合物。没有其他极端微生物能像嗜金属微生物这样与有毒金属亲密互动,这使它们成为环境修复、工业应用及天体生物学突破的关键所在。
- 耐辐射微生物在高辐射环境中(如核废料场、宇宙射线辐射的外太空或放射性矿藏)能旺盛生长。诸如耐辐射奇球菌等耐辐射微生物,凭借超高效的 DNA 修复酶,可承受高于人类致死剂量数千倍的辐射。这些特性有望推动新型癌症疗法、防护性抗氧化剂及 DNA 修复机制的研发。耐辐射微生物正被改造用于放射性污染治理:硫还原地杆菌能还原铀元素,降低其在地下水中的溶解度;耐辐射赤细菌可在核反应堆冷却剂中存活,分解有毒同位素;科研人员正尝试培育“超级耐辐射微生物”用于切尔诺贝利/福岛式核污染清理。
专利申请占比最高的极端微生物是嗜酸微生物(约 11%)和嗜热微生物(约 9%)
- 嗜酸微生物:这类微生物在金属提取、酸性矿山排水修复以及稀土元素回收(例如从废弃智能手机和电池中)方面具有关键作用。这使它们成为可持续采矿和循环经济战略中的关键参与者。嗜酸微生物能产生耐酸酶,这些酶在生物燃料生产(适应低 pH 的纤维素酶、木聚糖酶)、食品工业以及制药领域(例如从酸性热泉中发现新型抗生素)具有重要应用价值。在严苛的工业环境下,它们所产的酶通常比传统酶表现更卓越。对嗜酸菌的研究连接了微生物学、天体生物学、工业技术与环境科学,使其成为当今最具影响力的极端微生物类群之一。
- 嗜热微生物(通常在 45–80°C 环境中繁盛)与超嗜热微生物(最适生长温度 ≥80°C)因其独特的生物学特性及工业应用价值,已成为极端微生物研究的核心焦点。它们能产生在高温环境下仍保持活性的耐热极端酶。这使它们在聚合酶链式反应 (PCR) 中变得极为重要。如前所述,源自水生栖热菌的 Taq 聚合酶为 DNA 扩增技术带来了革命性突破。此外,它们还在医学和制药领域具有重要应用价值,例如诊断技术和药物合成中使用的热稳定性酶类。嗜热微生物能产生新型抗菌化合物,助力抗生素研发;同时它们对癌症研究至关重要,通过研究嗜热微生物中的热休克蛋白 (HSP),可深化对细胞应激反应机制的理解。
在可再生能源与废弃物处理研究中,嗜热微生物被广泛应用于生物燃料与生物氢能生产、沼气增效以及极端环境生物修复等领域。耐热酶在食品工业中显著优化了酿造、烘焙及乳制品生产工艺;而嗜热蛋白酶与脂肪酶通过提升热水洗涤的去污效能,造就了更卓越的洗涤剂产品。鉴于其广泛的应用领域,嗜热微生物在专利文献和商业用途中的高频出现也就不难理解了。
我们通过分析 CAS 内容合集,深入探究了极端酶及生物分子相关研究的发展趋势(参见图 3):

图 4 显示了不同类别的嗜极生物与所产生的极端酶类型的相对共存情况的热图:

氧化酶是与极端微生物相关文献数量最多的酶
氧化酶因其生态重要性、工业应用潜力以及对极端环境的独特适应性,已成为当前研究最深入的极端酶类别之一。许多极端微生物(如嗜热微生物、嗜碱微生物和嗜盐微生物)在缺氧或极端环境中依赖氧化酶进行呼吸作用。此外,部分氧化酶能协助极端微生物在严苛环境中应对氧化应激。其热稳定性至关重要,源自嗜热菌的氧化酶在高温下仍保持活性,这一特性在生物燃料生产、食品加工及废物处理领域具有广泛应用价值。其对极端 pH 值、高盐度及有机溶剂的耐受性,在严苛的工业流程中同样至关重要。
极端微生物氧化酶通常通常具有经过修饰的蛋白质结构,例如嗜盐微生物中的增强离子键或嗜热微生物中的紧凑折叠结构,从而使其更加稳健。对这些适应机制的研究,为从生物修复到医学等领域的酶工程应用提供了关键理论基础。
极端微生物漆酶、核酸酶、糖苷酶及过氧化氢酶展现出最显著的增长趋势
极端微生物漆酶在严苛工业环境中展现出独特稳定性,其卓越性能超越普通酶类:耐热性(70-100° C活性,适用于生物燃料与纸浆加工)、耐酸碱性(在强酸/强碱废液中保持活性)及有机溶剂耐受性(可在制药合成所需的有机溶剂中发挥作用)。这一特性使它们比许多其他极端酶更具工业应用价值,因为后者往往不具备如此全面的多重胁迫耐受性。宏基因组学、定向进化与 CRISPR 基因编辑技术的最新突破,使得从不可培养的极端微生物中发掘新型漆酶,并为其定制特定工业需求成为可能。与其他极端酶相比,这一优势显著加快了它们的开发进程。
近年来,另一类酶,核酸酶,近年来获得了极大关注,其在生物技术与医学应用领域的发展势头已可与漆酶及其他极端酶相媲美。它们能在极端环境下保持精确的 DNA/RNA 切割功能,这一特性使其在基因编辑、诊断技术和合成生物学等前沿领域变得不可或缺。极端微生物核酸酶(如热稳定 Cas9 变体、TaqI 限制性内切酶)正在彻底改变基因工程领域,其热稳定性使其能在高温 PCR 及基因编辑流程中发挥作用。耐盐与耐溶剂核酸酶使得在非标准实验室条件下(如直接环境样本分析)进行 DNA 操作成为可能。经过工程化改造的变体(包括源自火球菌属或栖热菌属的核酸酶)显著提升了 CRISPR-Cas 系统的精准度与运行效率。例如,嗜热栖热菌核酸酶被用于热启动 PCR 技术,可有效防止非特异性扩增。
极端微生物核酸酶在分子诊断与床旁检测领域具有不可替代的价值。热稳定性核酸酶是快速检测 DNA/RNA 的关键组分(如 COVID-19 逆转录聚合酶链反应),而嗜盐核酸酶能在高盐诊断缓冲液中保持活性,显著提升现场检测试剂的保质期。耐酸碱核酸酶能够从复杂样本中高效提取 DNA。极端微生物核酸酶的其他关键应用领域还包括:生物修复与抗生物膜技术、合成生物学与 DNA 数据存储,以及制药和抗病毒应用。
源自极端微生物的糖苷酶(或称糖苷水解酶)近期重要性显著提升,因其在极端条件下分解复杂碳水化合物的独特能力,为生物精炼、医药、食品科技及合成生物学等领域开辟了新应用前景。极端微生物糖苷酶已成为植物生物质转化为生物燃料和生化制品领域的技术颠覆者,其耐热特性 (70–100°C) 能够在工业条件下高效实现木质纤维素的糖化处理。其耐酸碱特性使其能在预处理工艺(如蒸汽爆破、酸解)中进行水解反应,而耐溶剂性则允许其在离子液体中用于生物质分解。
这些糖苷酶显著提升了食品加工效率并推动新产品研发:嗜热酸 α-淀粉酶(如源自硫化叶菌)可强化玉米糖浆生产中的高温淀粉液化工艺;冷适应 β-半乳糖苷酶(源自嗜冷微生物)能以更低能耗生产无乳糖牛奶;而嗜盐糖苷酶则能在高盐条件(如酱油、泡菜)下稳定发酵食品品质。嗜极糖苷酶在废物管理中也非常重要,因为在欧盟,食品和农业废物的酶促回收已成为强制要求。
源自极端微生物的过氧化氢酶(极端过氧化氢酶)作为另一类极端酶正快速获得关注,因其在需要高效降解过氧化氢 (H₂O₂) 的关键场景中展现出卓越活性。它们在高温、极端 pH、高盐度和氧化应激条件下仍能保持活性,这为新一代生物技术、医学和环境应用开辟了新途径。其热稳定性 (60–120°C) 在纺织漂白、造纸加工和食品灭菌过程中的过氧化氢去除环节发挥着关键作用。其耐酸碱性 (pH 3-11) 使其能在牛仔布漂白 (pH 10-11) 和乳品加工 (pH 6-7) 过程中保持酶活性不丧失。此外,其有机溶剂耐受性在生物燃料生产中得到应用,该过程中 H₂O₂ 是副产物。
过氧化氢酶在生物医学领域的新兴应用包括:利用极端过氧化氢酶降解慢性伤口中的过氧化氢以促进愈合;加速组织修复;用于阿尔茨海默病与帕金森病等氧化应激障碍的潜在抗氧化治疗;以及隐形眼镜清洁,嗜盐过氧化氢酶能消除消毒液中过氧化氢引发的眼部刺激。
极端微生物产生的胞外多糖已成为最有价值的微生物产品之一
这些由极端微生物合成的复合糖类具有独特性质,其性能优于传统多糖类物质。它们具备无与伦比的特性:卓越的热稳定性(如栖热菌属胞外多糖耐受 130°C高温)、宽广的耐化学性(适应 pH 0.5-13 环境及有机溶剂)、极强的持水能力(吸水量达自重 1000 倍)以及抗辐射性能。例如,酸热硫化叶菌胞外多糖在 95°C 高温与 pH 2 的强酸环境下仍能保持黏稠状态,性能超越所有商业增稠剂。
它们在医疗应用领域也已取得显著成果。盐单胞菌胞外多糖制成的水凝胶敷料可使伤口愈合时间缩短 40%,有效预防生物膜形成(对耐甲氧西林金黄色葡萄球菌抑制率达 90%),并在高盐环境中实现自灭菌。在药物递送领域,热球菌胞外多糖纳米颗粒能完整耐受胃酸侵蚀,在精准体温控制下释放药物,并于 72 小时后完全生物降解。最后在抗癌治疗中,红嗜热菌胞外多糖展现出 60% 的肿瘤生长抑制率,对健康细胞零毒性,并能与免疫治疗药物产生协同增效作用。
为了可视化与极端微生物相关的主要概念,以及具体类型、研究主题和应用,我们使用了基于文献发布情况的 TrendScape 地图(见图 5):

极端微生物的生物技术和工业应用
生物医学应用:
源自极端微生物的生物活性化合物与酶类,凭借其卓越稳定性及创新作用机制,正为制药与生物医学领域带来革命性突破。它们对 CRISPR-Cas9 技术的发展起到了关键作用。虽然源自化脓链球菌的经典 Cas9 存在稳定性局限,但经过热适应改造的 Cas 酶(如嗜热栖热菌 Cas9)能在高温条件下保持活性。源自嗜酸微生物的酶类还能在酸性环境(如肠道微生物组工程)中实现基因编辑。诸如耐辐射奇球菌等耐辐射微生物,为提升 CRISPR 基因编辑的精准度、降低脱靶效应提供了新型 DNA 修复模板。
生物大分子与极端酶正在心血管研究领域催生突破性发现。例如,源自地芽孢杆菌等嗜热微生物的纤溶酶,相较于现有溶栓药物具有更高的温度稳定性和更长的循环半衰期,能更高效地溶解血栓。来自嗜辐射微生物的抗氧化蛋白能够中和氧化应激,这是动脉粥样硬化和心力衰竭的关键驱动因素。极端微生物脂质在心血管药物递送领域也展现出应用潜力。例如,源自嗜热微生物的耐热脂质体可提升药物向动脉粥样硬化斑块的靶向递送效率,而来自南极微生物的冷适应膜转运蛋白则能助力药物更有效地穿透钙化动脉。
极端酶还能靶向蛋白质聚集体,这使得它在应对阿尔茨海默病、帕金森病及肌萎缩侧索硬化等神经退行性疾病方面具有潜在革命性意义。嗜热蛋白酶(例如源自水生栖热菌的酶类)不仅能高效降解 β-淀粉样蛋白和 tau 蛋白纤维,其效率超越人体自身酶类,更能耐受大脑的氧化应激环境。低温适配分子伴侣通过维持低浓度下的蛋白质稳定性,有效阻止帕金森病中 α-突触核蛋白的错误折叠。据报道,激烈火球菌蛋白酶在转基因小鼠模型中可使淀粉样斑块减少 70%。
除了这些生物医药应用外,嗜高温微生物如耐伽玛热古菌已被发现能产生具有抗菌和抗癌特性的多酮类化合物,可作用于多药耐药病原体和癌细胞系。极端酶还被应用于药物合成以及传感器与诊断工具的研发。
例如,源自嗜冷微生物的低温活性酶正被整合到生物传感器中,用于低温临床环境下的生物标志物检测,如糖尿病葡萄糖监测。诸如源自盐沼盐杆菌的细菌视紫红质等嗜盐蛋白,已被应用于光激活诊断系统。这些应用案例充分证明,极端微生物具有革新生物医学诸多领域的潜力。
环保应用:
生物修复技术利用微生物降解或消除环境污染物,正日益成为极端微生物的重要应用领域,尤其在常规微生物无法存活的极端环境中展现出独特优势。
- 重金属修复:包括嗜酸氧化亚铁硫杆菌在内的嗜酸微生物,可通过生物浸出技术从采矿废料中提取铜、铀等重金属,有效降低环境毒性。来自高盐湖的嗜盐微生物(如伸长盐单胞菌)能富集含盐工业废料中的镉等重金属,有效修复污染场地。
- 塑料与异生物质降解:最新研究揭示了极端微生物降解合成污染物的卓越能力。例如,一项 2024 年研究发现:嗜冷性 Ideonella sakaiensis 菌株能产生低温活性 PET 降解酶,这类酶可在寒冷环境中分解聚对苯二甲酸乙二醇酯 (PET) 塑料,为塑料废弃物治理提供了可持续解决方案。
生物燃料和可再生能源应用:
极端微生物通过在极端条件下实现高效的生物燃料合成与生物质转化,为可持续能源生产提供了关键技术支持。
- 生物乙醇生产:诸如热纤梭菌等嗜热菌能分泌纤维素酶,将木质纤维素生物质分解为可发酵糖类用于生物乙醇制备。其高温活性不仅能降低污染风险,还可加速水解过程,从而有效降低生产成本。
- 沼气与氢能:产甲烷古菌(如嗜热自养甲烷杆菌)在高温厌氧消化器中活跃生长,将有机废弃物转化为甲烷用于沼气生产。来自深海环境的嗜压细菌(如深渊希瓦氏菌)正被探索用于高压条件下开发生物氢能,为实现清洁能源提供了新途径。
- 藻类生物燃料:嗜盐微藻(如杜氏盐藻)在高盐度条件下积累脂质,并将其加工成生物柴油。它们对盐碱环境的适应能力使其能够在非耕地上种植,从而保护淡水资源。
食品饮料加工应用:
极端微生物通过提供能在工业食品生产极端环境下保持活性的酶类,显著提升了食品加工效率。
- 乳制品与饮料加工:嗜冷蛋白酶和脂肪酶被应用于奶酪熟化与果汁澄清工艺,其低温操作特性可有效保持产品风味与营养品质。例如,假交替单胞菌来源的酶类能在冷藏条件下提升乳蛋白水解效率。
- 淀粉和糖加工:来自地衣芽孢杆菌的嗜热淀粉酶在高温加工过程中水解淀粉,用于生产饮料和糖果的糖浆。这些酶确保高产量并缩短加工时间。
- 发酵工艺:源自盐杆菌属的嗜盐酶能促进鱼露、酱油等高盐发酵过程,在强盐环境下有效提升风味品质。
农业应用:
极端微生物通过生物农药、土壤修复和促进植物生长,为可持续农业做出贡献。
- 土壤修复:诸如里约酸硫杆菌等嗜酸菌能活化污染土壤中的重金属,有效恢复农田耕作功能。其基于硫元素的代谢机制可有效修复受采矿污染的区域。
- 植物抗逆性提升:嗜盐菌渗透保护剂(如源自伸长盐单胞菌的四氢甲基嘧啶羧酸)可增强作物对盐碱与干旱的耐受能力,有效提高干旱地区农作物产量。2025 年的田间试验表明,经四氢甲基嘧啶羧酸处理的盐碱地小麦产量显著提升。
工业材料和工艺:
极端微生物已广泛应用于纺织、皮革和化妆品等行业,其强效酶类与活性成分在严苛的工业加工环境中展现出卓越性能。
- 纺织加工:源自嗜碱芽孢杆菌的碱性蛋白酶可在碱性环境下去除蛋白质污渍并辅助染色,从而提升纺织品质量,减少水资源消耗。
- 皮革鞣制:嗜盐酶与嗜碱酶能在高盐或高 pH 的鞣制工艺中实现对生皮的脱毛与脱脂处理。同时,它们能够替代有毒化学制剂。
- 化妆品领域:来自杜氏盐藻等嗜盐微生物的四氢甲基嘧啶羧酸和 β-胡萝卜素,凭借其在高盐配方中的稳定性,被用于保湿剂与防晒产品,能有效保护皮肤免受紫外线伤害与脱水问题。
极端微生物的未来发展方向
尽管潜力巨大,极端微生物的广泛应用仍面临诸多挑战。培养需特殊条件的微生物以及酶的高昂纯化成本是两个重要障碍,同时还有生产规模化的问题。宏基因组学与合成生物学正通过将极端微生物基因在大肠杆菌等易操作宿主中表达,来应对这些难题。
高通量筛选与人工智能驱动的生物勘探技术的进步,正在持续加速新型极端酶及活性化合物的发现进程。将极端微生物衍生技术与纳米技术、绿色化学相结合,有望显著提升其效能并拓展应用边界。
为了充分发挥极端微生物的潜在益处,研究人员应重点关注以下几个关键方向:
- 探索未充分研究的极端环境:对研究较少的极端环境(如深层地下、极干旱沙漠和高辐射区)进行调查,可能会发现具有前所未有的适应能力的新型嗜极生物。
- 生存策略的机理研究:应采用先进的基因组学、蛋白质组学和代谢组学方法,系统解析极端微生物抗逆性背后的分子作用机制。
- 合成生物学与酶工程:通过基因改造和定向进化技术,对极端微生物来源的生物分子进行优化,将显著提升其在工业领域的应用潜力。
- 天体生物学与太空探索:应进一步研究极端微生物作为潜在外星生命的模型,特别是在火星模拟环境及木卫二、土卫二等冰质卫星的探测任务中。
- 跨学科协作:融合微生物学、生物信息学、材料科学与工程学,将加速科学发现与技术应用的进程。
随着技术不断进步,极端微生物将持续重塑我们对生命适应性的认知,并为应对人类面临的重大挑战带来创新性解决方案。
更多信息,请参阅我们的期刊文章“极端微生物:从边缘生命中开发生物活性化合物与生物技术创新”(Extremophiles: Unlocking Bioactive Compounds and Biotechnological Innovation from Life at the Edge)。