Las nuevas tecnologías de baterías proliferan a medida que aumenta la demanda de soluciones de almacenamiento de energía seguras y eficaces. Las baterías de estado sólido (SSB) representan un gran avance en la tecnología de almacenamiento de energía y tienen el potencial de superar varias limitaciones de las baterías tradicionales de iones de litio (LIB). Al reemplazar los electrolitos líquidos o en gel inflamables por materiales sólidos como cerámica, polímeros o sulfuros, las baterías de estado sólido ofrecen una mayor seguridad, una estabilidad térmica superior y densidades energéticas considerablemente más altas, que alcanzan los 500 Wh/kg en comparación con los 250 Wh/kg de los sistemas convencionales. La presencia de un electrólito sólido no solo permite utilizar ánodos de metal de litio y cátodos de gran capacidad, sino que también reduce los riesgos de alta inflamabilidad, formación de dendritas, descomposición electrolítica a altos voltajes y fugas que afectan a las baterías basadas en electrolitos líquidos.
Con la capacidad de durar más de 1000 ciclos (frente a los 500 ciclos de las LIB típicas), las baterías de estado sólido también prometen una vida útil más larga. Su tamaño más reducido y su potencial para un diseño más compacto que los LIB los hacen ideales para su uso en vehículos eléctricos (VE), aparatos electrónicos portátiles, dispositivos médicos como marcapasos y aplicaciones aeroespaciales.
Examinamos CAS Content CollectionTM, el mayor repositorio de información científica catalogado por humanos, para comprender mejor el panorama de la investigación de las baterías de estado sólido, y encontramos un claro cambio de la investigación en etapa temprana al interés científico y comercial generalizado (véase la Figura 1).

Figura 1: Tendencias de las publicaciones en el campo de las baterías de estado sólido. Los datos de 2025 son hasta julio. Fuente: Colección de contenidos CAS.
A principios de los 2000, la actividad era mínima, lo que indica que las baterías de estado sólido estaban limitadas por una investigación básica. Sin embargo, alrededor de 2017, los artículos académicos y patentes aumentaron considerablemente, lo que reflejó la creciente madurez tecnológica, la demanda del mercado y el reconocimiento de esta tecnología como solución a las principales limitaciones de las baterías convencionales de iones de litio.
Ahora, el número ligeramente superior de patentes indica que el campo va más allá de la investigación académica y pasa a la comercialización, por lo que las empresas se aseguran la propiedad intelectual. El fuerte crecimiento de las patentes en los últimos años refleja la urgente demanda impulsada por el aumento de los vehículos eléctricos, el almacenamiento en red de las energías renovables y la necesidad de soluciones energéticas más seguras y de alto rendimiento. El impulso actual indica que las baterías de estado sólido han alcanzado un punto crítico de investigación e inversión, a menudo observado justo antes de grandes avances y adopción generalizada.
Asia lidera la innovación mundial en baterías
Para evaluar las iniciativas de comercialización dentro del sector SSB, identificamos las 15 principales empresas en función de su actividad de patentes (Figura 2a). Las empresas japonesas, como Toyota y Panasonic, lideran gracias a sus amplias carteras de patentes, lo que refleja la temprana inversión estratégica de Japón en tecnología SSB y su fuerte alineación con la industria automovilística. La presencia de otras grandes empresas asiáticas, como LG Chem, Samsung SDI y CATL, refuerza aún más el liderazgo de Asia en la Innovación mundial en baterías (véase la Figura 2a).
Las tendencias de la actividad de patentes comerciales en diferentes países revelan dinámicas cambiantes de innovación en el campo de las SSB (Figura 2b). Aunque Japón ha mantenido un liderazgo constante mediante inversiones regulares e investigaciones a largo plazo, el rápido ascenso de China en los últimos años supone un cambio considerable. Este aumento se debe no solo a las firmas establecidas, sino también a una ola de nuevos participantes que solicitan patentes de forma activa.
En cambio, la actividad de patentes relativamente menor de Estados Unidos y Alemania, a pesar de sus sólidas industrias automovilísticas y tecnológicas, sugiere prioridades estratégicas diferentes o métodos alternativos para la protección de la propiedad intelectual en el ámbito de las baterías de estado sólido (SSB).
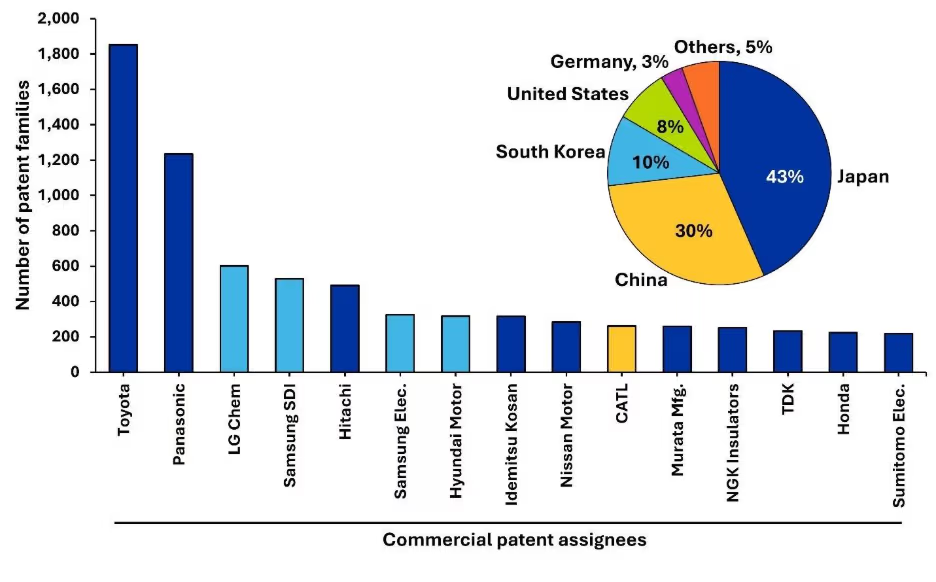
Figura 2a: Principales entidades comerciales identificadas por su número de publicaciones de patentes relacionadas con las baterías de estado sólido. El gráfico circular insertado muestra el volumen de patentes comerciales publicadas por países/regiones.
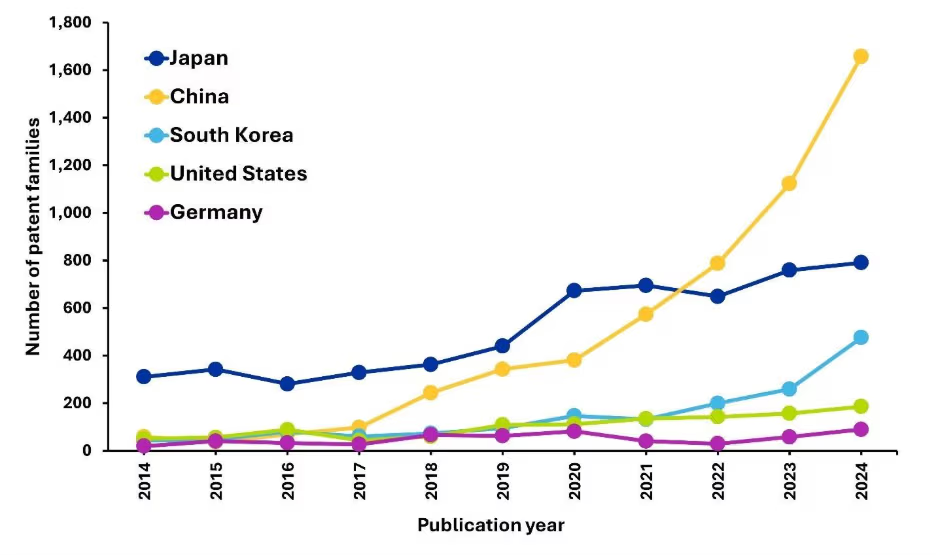
Figura 2b: Tendencias anuales para las publicaciones comerciales de patentes en los países o regiones líderes.
Ventajas de los electrolitos de estado sólido
Los electrolitos de estado sólido (SSE) son el componente central de las baterías de estado sólido (SSB), ya que funcionan como sólidos conductores de iones que sustituyen a los electrolitos líquidos tradicionales. Permiten transportar iones de litio u otros iones metálicos entre los electrodos de la batería y, al mismo tiempo, separarlos físicamente, lo que garantiza tanto la conductividad iónica como el aislamiento eléctrico.
Una característica destacable de los electrolitos en estado sólido es su capacidad para facilitar el transporte de iones a través de sitios cristalográficos, en los que los iones móviles pueden comportarse como fases fluidas dentro de una matriz sólida. Esta característica estructural sustenta su alta movilidad iónica, que es esencial para un rendimiento eficiente de la batería. Los electrolitos en estado sólido también ofrecen ventajas significativas sobre los sistemas líquidos, como la no inflamabilidad, la no volatilidad, una resistencia mecánica superior y la resistencia a las temperaturas extremas. Estas características no solo mejoran la seguridad y la estabilidad, sino que también permiten el uso de ánodos de metal de litio, que pueden aumentar drásticamente la densidad energética y la vida útil de las baterías.
Los electrolitos de estado sólido pueden clasificarse en cinco tipos principales según su composición química: óxidos, sulfuros, polímeros, nitruros y haluros. Cada tipo presenta características estructurales y electroquímicas propias que influyen en su rendimiento. Los electrolitos de estado sólido orgánicos e inorgánicos se han estudiado exhaustivamente en los últimos años, lo que ha llevado a mejoras significativas en la conductividad iónica, la estabilidad y la compatibilidad con los electrodos.
Nuestro análisis de la Colección de contenidos de CAS con herramientas como CAS SciFinder® ilustra los 20 principales electrolitos de estado sólido categorizados por tipo (Figura 3a) y año (Figura 3b). Entre ellos, los electrolitos inorgánicos, principalmente óxidos y sulfuros, lideran el panorama de las publicaciones debido a su conductividad iónica superior, su mayor seguridad, la larga vida útil de sus ciclos y su excelente estabilidad térmica.
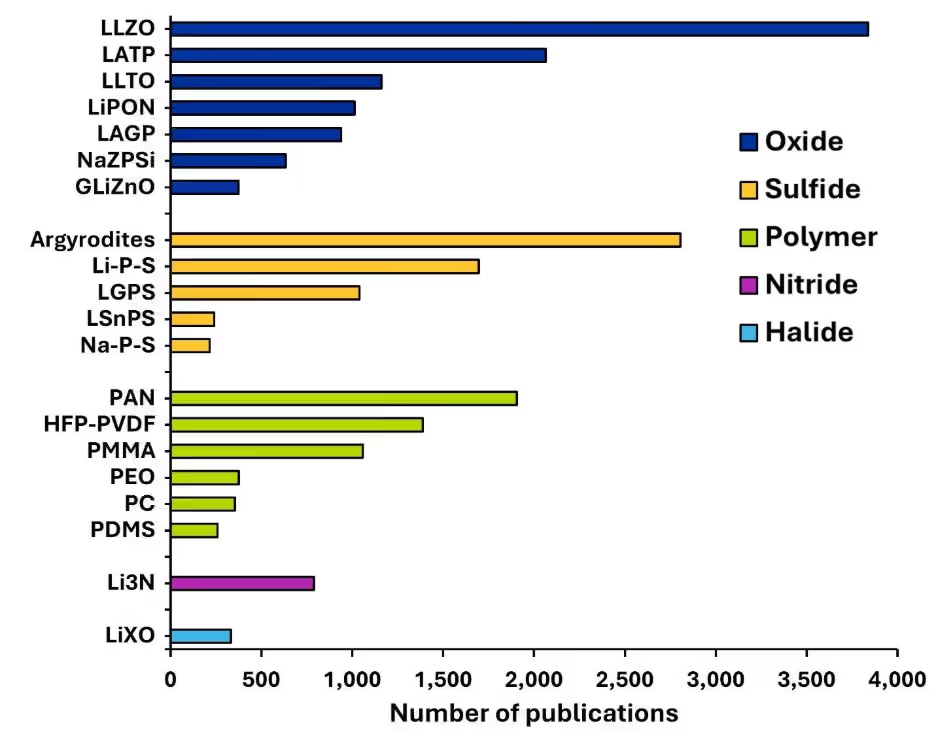
Figura 3a: Los 20 principales electrólitos de estado sólido categorizados por tipo dentro del volumen de publicaciones. Fuente: Colección de contenidos de CAS. Véase la nota al pie para las abreviaturas.
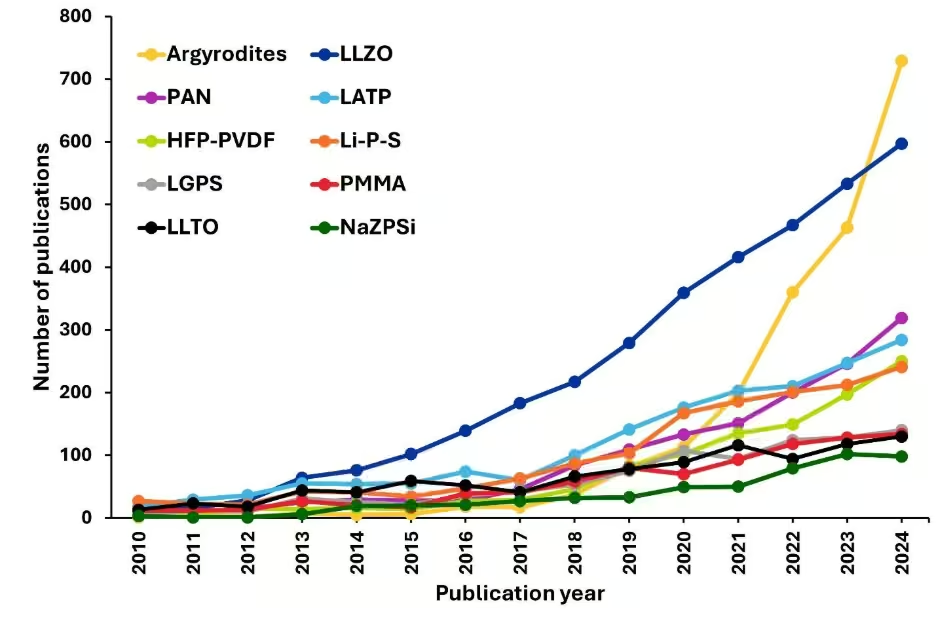
Figura 3b: Los 10 principales electrólitos de estado sólido clasificados por año dentro del volumen de publicaciones. Fuente: Colección de contenidos de CAS. Véase la nota al pie para las abreviaturas.
Electrolitos sólidos basados en óxidos
Los electrolitos de estado sólido basados en óxidos (SSE) están ampliamente estudiados para las baterías de estado sólido de próxima generación debido a su excelente estabilidad química, térmica y electroquímica. Estos electrolitos son estables frente al aire y la humedad, lo que facilita su manipulación y procesamiento. También soportan altas tensiones y temperaturas de funcionamiento sin descomponerse.
Como se muestra en la Figura 3, los principales tipos de electrolitos sólidos de óxido incluyen óxido de litio, lantano y zirconio, tipo granate (LLZO), fosfato de litio, aluminio y titanio, tipo NASICON (LATP), y óxido de litio, lantano y titanio, tipo perovskita (LLTO); todos ofrecen una alta conductividad iónica (típicamente 10⁻⁴ a >10⁻³ S/cm) y propiedades mecánicas robustas. El LLZO ofrece una amplia ventana de estabilidad electroquímica y buena compatibilidad con el metal litio, mientras que LATP es no tóxico, rentable y adecuado para los sistemas híbridos. El LLTO ofrece alta conductividad y una sólida resistencia mecánica, aunque su rendimiento depende de la microestructura cerámica.
A pesar de su potencial, los electrolitos basados en óxidos tienen varias limitaciones. Su estructura quebradiza y rígida dificulta su procesamiento y el contacto íntimo con los electrodos, lo que a menudo provoca una elevada resistencia interfacial y una transferencia iónica deficiente. Esta fragilidad también los hace vulnerables a agrietarse bajo estrés mecánico o cambios de volumen durante el ciclo. Además, su conductividad a temperatura ambiente suele ser inferior a la de los electrolitos a base de sulfuro.
La tecnología de LLZO puede sufrir sensibilidad a la humedad y formación de dendritas de litio, mientras que LATP y LLTO son inestables en contacto directo con el litio metálico debido a la reducción del Ti⁴⁺. Estos problemas pueden provocar cortocircuitos, aunque pueden mitigarse mediante ingeniería de interfaces y recubrimientos protectores. En general, los electrolitos de óxido se valoran por su excelente estabilidad, seguridad y compatibilidad con cátodos de alta tensión, lo que los convierte en candidatos adecuados para sistemas duraderos y térmicamente estables de baterías de litio de estado sólido.
Electrolitos de estado sólido basados en sulfuros
Los electrolitos a base de sulfuro son uno de los materiales más prometedores para las baterías de estado sólido de próxima generación debido a su conductividad iónica excepcionalmente alta, que puede alcanzar hasta 10-2 S/cm, comparable o incluso superior a la de los electrolitos líquidos. Sus estructuras, que normalmente contienen aniones a base de azufre, como el PS³³, crean una red flexible y altamente polarizable que permite una rápida migración de los iones de litio.
Otra ventaja importante de los electrolitos de sulfuro es su naturaleza blanda y deformable, que les permite mantener un excelente contacto interfacial con los cátodos y ánodos a una presión suave, algo difícil de conseguir con electrolitos de óxido quebradizos. También pueden procesarse a bajas temperaturas, lo que hace factible su fabricación a gran escala.
Como se ve en la Figura 3a, entre los electrolitos basados en sulfuros, las argiroditas, el litio-fósforo-azufre (Li-P-S) y el sulfuro de germanio-fósforo de litio (LGPS) son los principales candidatos para baterías de estado sólido. Los sistemas de argirodita (Li₆PS₅X, X = Cl, Br, I) y los sistemas Li–P–S (por ejemplo, Li₃PS₄, Li₇P₃S₁₁) ofrecen conductividades que van desde 10⁻⁴ hasta >10⁻³ S/cm, mientras que LGPS (por ejemplo, Li₁₀GeP₂S₁₂), un material tio-LISICON, alcanza hasta 10⁻² S/cm, rivalizando con los electrolitos líquidos.
A pesar de estas ventajas, los electrolitos a base de sulfuros también se enfrentan a obstáculos críticos. Son muy sensibles al aire y a la humedad, y cuando se descomponen liberan gas tóxico de sulfuro de hidrógeno (H₂S), lo que complica su manipulación y procesamiento. Su periodo de estabilidad electroquímica es breve, lo que provoca reacciones indeseables en los cátodos de alto voltaje y en los ánodos de metal de litio, que forman interfases resistivas y degradan el rendimiento con el tiempo. No obstante, con mejoras continuas mediante dopaje, recubrimientos superficiales e ingeniería de compuestos, los electrolitos sólidos basados en sulfuros siguen estando a la vanguardia de la investigación para obtener baterías de litio de estado sólido de alto rendimiento, seguras y energéticamente densas.
Electrolitos sólidos basados en polímeros
Los electrolitos basados en polímeros se utilizan en las baterías de estado sólido porque ofrecen una combinación única de flexibilidad, procesabilidad y seguridad de la que suelen carecer los electrolitos inorgánicos. A diferencia de los frágiles electrolitos de óxido, los electrolitos poliméricos son blandos y deformables, lo que les permite formar un contacto íntimo con los electrodos. También permiten obtener diseños de baterías finos, ligeros y flexibles, ideales para los dispositivos electrónicos portátiles y ponibles.
Entre los electrolitos sólidos basados en polímeros, el poliacrilonitrilo (PAN), el fluoruro de hexafluoropropileno-polivinilideno (PVDF-HFP) y el poli(metacrilato de metilo) (PMMA) son los materiales principales. Sin embargo, su conductividad iónica suele ser inferior a la de los electrolitos inorgánicos, especialmente a temperatura ambiente, y pueden sufrir una estabilidad electroquímica limitada a alto voltaje. Su excelente flexibilidad, compatibilidad interfacial y escalabilidad siguen convirtiendo a los electrolitos basados en polímeros en una opción atractiva y práctica para las aplicaciones de baterías de litio de estado sólido y flexibles.
Electrolitos de estado sólido basados en nitruros y haluros
Los electrolitos basados en nitruro y haluro son nuevas clases de materiales explorados para obtener LIB en estado totalmente sólido de alto rendimiento debido a su alta conductividad iónica y a su favorable estabilidad electroquímica. Como se observa en la Figura 3b, el nitruro de litio (Li₃N) y el LiXO (X= Cl, Br, I) son los únicos nitruros y haluros, respectivamente, entre los 20 principales electrolitos de estado sólido según las tendencias de publicación.
El Li₃N ofrece un transporte excepcionalmente rápido de iones de litio, con conductividades de hasta 10⁻³ S/cm a temperatura ambiente, debido a sus estructuras cristalinas abiertas que proporcionan vías continuas para la migración del Li⁺. También son térmicamente estables y compatibles con los ánodos metálicos de litio. El LiXO generalmente se combina con otros electrólitos sólidos o polímeros para mejorar el contacto interfacial y la flexibilidad mecánica.
Estos materiales también tienen importantes inconvenientes. Los electrolitos sólidos basados en nitruros y haluros son muy reactivos a la humedad y el aire, por lo que generan subproductos tóxicos y corrosivos como el amoníaco y el HCl, lo que complica su manipulación y procesamiento. A pesar de estas limitaciones, los electrolitos de nitruro y haluro siguen siendo vías prometedoras para desarrollar baterías de litio de estado sólido de alta densidad energética y térmicamente robustas, especialmente cuando se incorporan en sistemas de electrolitos compuestos.
Las argiroditas se convierten en la principal tecnología de electrolitos de estado sólido
Analizamos más a fondo las tendencias de publicación de los 10 principales materiales electrolitos de estado sólido y descubrimos un cambio fascinante en el método de investigación en estos últimos años (véase la Figura 3b). Los electrolitos tipo granate LLZO dominaron el campo hasta hace poco, atrayendo una atención constante de la comunidad investigadora debido a su excelente estabilidad frente al metal litio y su amplia ventana electroquímica. Sin embargo, en 2021 se produjo una transformación radical: las argiroditas basadas en sulfuros experimentaron un aumento explosivo en el interés de investigación que las ha impulsado hasta adelantar al LLZO para convertirse en el sistema de electrólitos sólidos más estudiado en 2024.
Este notable ascenso de las argiroditas refleja varias ventajas fundamentales que se han hecho evidentes para los investigadores. Si bien el LLZO requiere temperaturas de sinterización extremadamente altas y tiene problemas de contacto interfacial debido a su naturaleza cerámica, las argiroditas ofrecen una ductilidad mecánica que permite un contacto íntimo con los materiales de los electrodos mediante un simple prensado en frío. La estructura de sulfuros proporciona conductividades iónicas que superan con creces las de los sistemas a base de óxido a temperatura ambiente, lo que soluciona uno de los cuellos de botella clave para la comercialización de baterías de estado sólido.
Además, la compatibilidad del procesamiento de las argiroditas con la infraestructura existente de fabricación de baterías los ha hecho atractivos para su uso industrial, ya que pueden procesarse a temperaturas moderadas e incluso mediante métodos basados en soluciones. Por su parte, otros sistemas de electrolitos sólidos, como LATP y los electrolitos basados en polímeros, siguen atrayendo una atención constante, aunque más modesta, de los investigadores, lo que indica que si bien estos materiales siguen siendo relevantes, el campo ha identificado a las argiroditas como la opción más prometedora a corto plazo para crear baterías de estado sólido prácticas.
Combinaciones líderes de materiales de estado sólido
Nuestro análisis del panorama de publicaciones también exploró diferentes tipos de baterías de estado sólido (SSB) y los principales electrolitos de estado sólido (SSE) asociados a ellas (véase la Figura 4). Las LIB dominan el panorama con el mayor volumen de documentos: representan aproximadamente el 80 % de toda la actividad de investigación sobre baterías de estado sólido. La división casi equitativa entre publicaciones de revistas y familias de patentes indica que es un campo maduro que está transitando de la investigación básica a la comercialización.
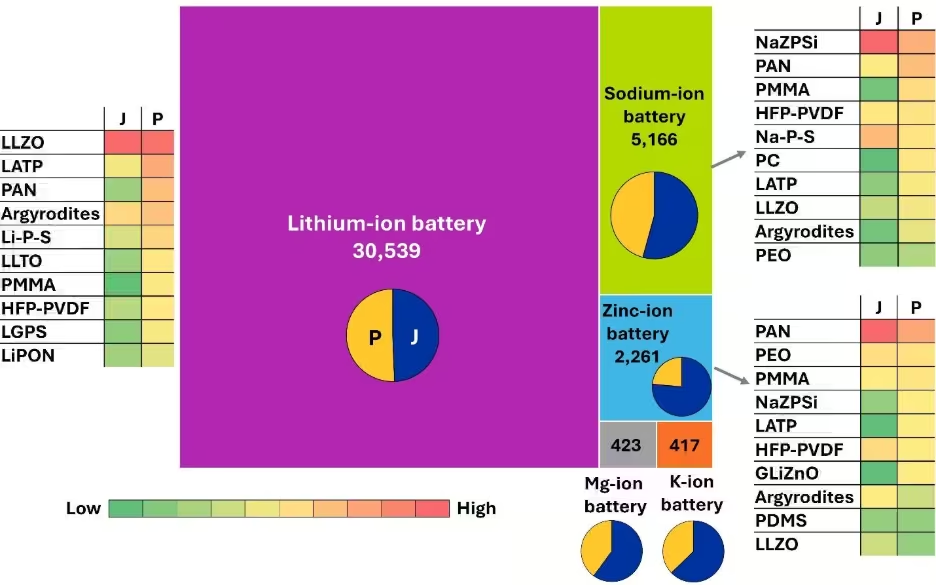
Figura 4: Distribución de la investigación de baterías de estado sólido en diferentes químicas de baterías y electrolitos de estado sólido asociados. Los gráficos circulares individuales indican la proporción de publicaciones de revistas (J) y patentes (P) para las respectivas baterías. Las tablas de mapas de calor muestran los electrolitos de estado sólido más prevalentes asociados con sus respectivas baterías de estado sólido. Fuente: Colección de contenidos CAS. Véanse las notas al pie para las abreviaturas.
Los principales electrolitos en estado sólido para los sistemas de litio muestran una interesante diversidad. Los electrolitos de LLZO y LATP a base de óxido son los que más atención reciben, seguidos de los electrolitos poliméricos como el PAN, las argiroditas a base de sulfuro y los sistemas Li-P-S. Esta variedad indica que el campo no ha convergido en una única tecnología ganadora, ya que se están explorando diferentes electrolitos de estado sólido para los distintos requisitos de aplicación.
Las baterías de estado sólido de iones de sodio ocupan el segundo lugar en volumen de documentos, aunque siguen muy por detrás de los sistemas de litio. La proporción entre patentes y publicaciones favorece ligeramente las publicaciones en revistas, lo que indica que este campo sigue estando más centrado en la investigación que en la comercialización. Las preferencias de electrolitos para los sistemas de sodio muestran diferencias notables con las del litio: el NazPsi (conductor superiónico de sodio) lidera el grupo, mientras que los electrolitos poliméricos (PAN, PMMA) ocupan un lugar destacado. También están presentes algunos electrolitos centrados en el litio, como el LATP y el LLZO, lo que indica que los investigadores están estudiando la compatibilidad cruzada entre los sistemas.
Las baterías de iones de zinc suponen la tercera opción relevante y muestran una proporción mucho mayor de publicaciones en revistas en comparación con las patentes. Esta distribución mayoritaria en revistas sugiere que las baterías de estado sólido de zinc aún se encuentran en la fase de investigación básica y que los investigadores intentan entender y optimizar estos sistemas antes de su desarrollo comercial. El panorama de los electrolitos de estado sólido para las baterías de zinc está dominado por los electrolitos poliméricos (PAN, PEO, PMMA), lo que es lógico, dada la compatibilidad del zinc con los sistemas acuosos y cuasisólidos.
Las nuevas químicas de las baterías, como el magnesio y el potasio, muestran una actividad equilibrada de investigación y patentes a pesar de tener cifras mundiales más bajas, lo que indica un desarrollo en fase temprana pero prometedor. Estos sistemas podrían representar alternativas futuras a medida que los investigadores traten de diversificar fuera del litio, sobre todo para aplicaciones específicas en las que sus propiedades únicas ofrezcan ventajas.
Las intensidades del mapa de calor entre diferentes electrolitos, mostradas en la Figura 4, indican que, mientras que ciertos electrolitos muestran versatilidad en varios tipos de batería, otros siguen siendo específicos de la química. Esto pone de manifiesto la importancia del desarrollo de electrolitos de estado sólido adaptado a los requisitos propios de cada sistema de batería.
Las tendencias en patentes muestran aplicaciones prometedoras para las baterías de estado sólido
Nuestro análisis de los volúmenes de publicación en distintos sectores de aplicaciones de baterías de estado sólido (SSB) revela patrones distintos en el proceso de la investigación y la madurez de comercialización (véase la Figura 5).
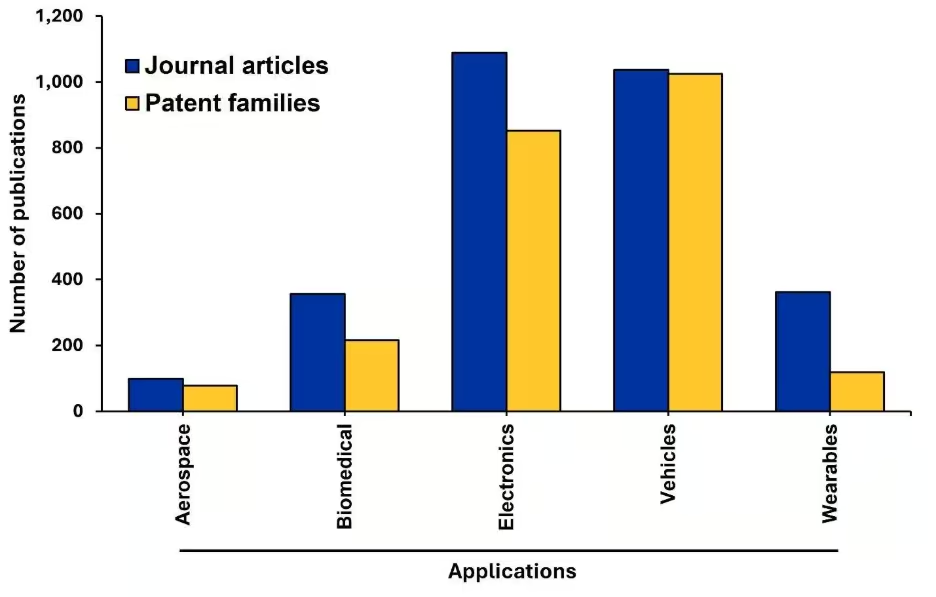
Figura 5: Número de publicaciones en revistas y patentes para aplicaciones de pilas de estado sólido. Fuente: Colección de contenidos de CAS.
La electrónica y los vehículos dominan claramente el campo, ya que representan aproximadamente el 75 % de toda la investigación de aplicaciones. Sin embargo, sus trayectorias de desarrollo difieren significativamente. La electrónica muestra una brecha notable entre las publicaciones de revistas y las patentes, lo que indica que hay desafíos en la investigación básica en curso para adaptar estas baterías a la electrónica de consumo. Esto podría estar relacionado con la miniaturización, el coste o la complejidad de la fabricación. Por el contrario, el sector de los vehículos muestra una notable paridad entre publicaciones y patentes. Estos resultados indican que es un campo maduro en transición del laboratorio al mercado, impulsado por el crecimiento de los vehículos eléctricos y la necesidad urgente de baterías más seguras y de mayor densidad energética.
Los sectores emergentes de aplicaciones muestran potencial, pero siguen centrándose predominantemente en la investigación. Las aplicaciones biomédicas demuestran un interés académico considerable con una actividad limitada en patentes, lo que sugiere que los investigadores aún exploran la viabilidad fundamental en lugar la comercialización. Esto podría deberse a los estrictos requisitos regulatorios y los problemas de biocompatibilidad propios de los dispositivos médicos.
Los dispositivos portátiles muestran una brecha aún más pronunciada entre la investigación y las patentes, lo que indica que, si bien el concepto de baterías de estado sólido flexibles y seguras para la tecnología portátil es interesante desde el punto de vista académico, aún quedan importantes obstáculos técnicos para la viabilidad comercial.
El sector aeroespacial cuenta con una distribución casi equitativa de artículos de revistas y patentes. Esta ratio equilibrada, a pesar de un volumen total de documentos más bajo, refleja el patrón observado en el sector de los vehículos. Indica una participación comercial activa impulsada por varias ventajas técnicas atractivas de las baterías de estado sólido en las aplicaciones aeroespaciales, como su no inflamabilidad y su mejor rendimiento a grandes altitudes.
Desafíos y oportunidades con las baterías de estado sólido
Aunque las baterías de estado sólido (SSB) son muy prometedoras, varios obstáculos impiden su comercialización a gran escala. Una limitación técnica importante es la conductividad iónica relativamente baja de muchos electrólitos sólidos a temperatura ambiente en comparación con los líquidos. Los electrolitos basados en óxido como el LLZO requieren altas temperaturas de sinterización para obtener microestructuras densas, lo que hace que el procesamiento sea complejo y costoso. Los electrolitos de sulfuro ofrecen una alta conductividad, pero son sensibles al aire y a la humedad, por lo que requieren condiciones inertes.
Otro obstáculo es la inestabilidad en las interfaces electrodo-electrolito. A diferencia de los electrolitos líquidos, el contacto entre sólidos y sólidos a menudo da como resultado un mal contacto interfacial y una alta resistencia. Las reacciones químicas en estas interfaces pueden formar capas resistivas, especialmente con el metal de litio, que empeoran con los ciclos y degradan el rendimiento. La rigidez mecánica de los electrolitos sólidos contribuye además a la tensión, las fisuras y la deslaminación durante los cambios de volumen, lo que aumenta la impedancia y el deterioro del rendimiento.
La formación de dendritas de litio sigue siendo un problema, ya que los filamentos de litio pueden propagarse a través de defectos microestructurales en los electrolitos de estado sólido en altas densidades de corriente y provocar cortocircuitos. Además, la producción a gran escala es costosa, ya que el procesamiento de estos materiales requiere altas temperaturas, alta presión, ingeniería precisa y condiciones secas (especialmente para los sulfuros). Estos obstáculos materiales y de integración, junto con los problemas de reciclabilidad y la inmadurez de las cadenas de suministro, dificultan la transición de los prototipos a escala de laboratorio a las baterías de estado sólido a escala industrial.
A pesar de todo ello, las baterías de estado sólido están evolucionando hacia un futuro en el que el diseño de materiales, la ingeniería de interfaces y el procesamiento escalable converjan para ofrecer un almacenamiento de energía más seguro, denso y duradero. La próxima generación de estas baterías contará con electrolitos híbridos y compuestos que combinen la alta conductividad de la cerámica con la flexibilidad de los polímeros, para garantizar interfaces estables e integridad mecánica durante los ciclos. Los avances en la fabricación a baja temperatura, como la sinterización en frío y el procesamiento asistido por líquidos transitorios, harán que la fabricación sea más eficaz y compatible con la producción a gran escala.
Los prototipos recientes reflejan este auge: Chery ha presentado un módulo de batería de estado sólido con una densidad energética de 600 Wh/kg con un alcance de 1300 km, más del doble que las baterías de iones de litio convencionales. Del mismo modo, Sunwoda ha presentado una batería de polímero de estado totalmente sólido con 400 Wh/kg y un ciclo de vida de 1200 ciclos a presión ultrabaja. Esto podría dar a los VE una autonomía de más de 1000 km y 1200 ciclos, lo que permitiría más de una década de uso, calculando una conducción anual de 20 000 km.
Mientras tanto, los métodos basados en datos y el descubrimiento de materiales asistido por IA acelerarán la optimización y el control de calidad en este campo. Además del litio, los conceptos de estado sólido se extienden a otros iones metálicos como los sistemas de sodio, zinc, magnesio y potasio, lo que amplía la sostenibilidad y la accesibilidad de los recursos. Estos avances continuos y los métodos híbridos están acabando poco a poco con las diferencias de rendimiento entre la tecnología de baterías tradicional y las nuevas opciones de estado sólido.
Aunque la implementación a gran escala aún puede estar a años de distancia, la convergencia de avances científicos e industriales indica que las baterías de estado sólido están en camino de transformar el almacenamiento de energía en diferentes industrias en la próxima década.
Abreviaturas utilizadas: LLZO, óxido de litio, lantano y circonio; LATP, fosfato de litio, aluminio y titanio; LLTO, óxido de litio, lantano y titanio; LiPON, oxinitruro de litio y fósforo; LAGP, fosfato de litio, aluminio y germanio; NaZPSi, silicato de fosfato de circonio y sodio; GLiZnO, óxido de litio, germanio y zinc; Li-P-S, litio, fósforo y azufre; LGPS, sulfuro de litio y fósforo y germanio; LSnPS, sulfuro de litio y fósforo y estaño; Na-P-S, sulfuro de sodio y fósforo; PAN, poliacrilonitrilo; PVDF-HFP, fluoruro de hexafluoropropileno y poli(vinilideno); PMMA, polimetacrilato de metilo; PEO, óxido de polietileno; PC, policarbonato; PDMS, polidimetilsiloxano.