A principios de 2025, los científicos anunciaron que habían rescatado a los lobos gigantes de la extinción con tres cachorros de lobo modificados genéticamente. El entusiasmo de los medios de comunicación puede haber ocultado algunos detalles clave, en particular la modificación de los genomas de los lobos grises existentes con solo ciertas ediciones genéticas de los lobos gigantes, pero demostró las posibilidades de las tecnologías genéticas avanzadas para revivir genes que se creían perdidos para siempre.
Sin embargo, en lugar de recuperar especies extintas, una aplicación más inmediata y manejable de esta tecnología es la desextinción molecular, que consiste en la resurrección selectiva de genes, proteínas o vías metabólicas extintos. Este campo emergente aprovecha dos disciplinas científicas principales: la paleogenómica, el estudio del ADN antiguo (ADNa), y la paleoproteómica, el análisis de proteínas antiguas conservadas en restos fosilizados y subfósiles.
Estos enfoques permiten a los científicos explorar la historia evolutiva en busca de nuevos compuestos bioactivos que podrían revolucionar la medicina, la biotecnología y la biología sintética. En concreto, la desextinción molecular podría abordar los problemas de resistencia a los antibióticos al proporcionar fuentes no convencionales de moléculas antimicrobianas.
Los recientes avances tecnológicos han impulsado la desextinción molecular desde la especulación teórica hasta la realidad experimental. La secuenciación de nueva generación (NGS) y la secuenciación de lectura larga de tercera generación han mejorado drásticamente la recuperación del ADN antiguo fragmentado, mientras que la espectrometría de masas de alta resolución y el modelado bioinformático de proteínas permiten a los investigadores reconstruir secuencias de proteínas antiguas y predecir sus funciones.
Con los avances en biología computacional e inteligencia artificial, la identificación de moléculas favorables ha pasado de ser un proceso en gran medida aleatorio a una metodología más deliberada y basada en datos, en la que los investigadores pueden centrarse en características moleculares específicas basándose en un análisis exhaustivo de datos para predecir su eficacia potencial.
Tendencias de investigación en la desextinción molecular
Analizamos la CAS Content CollectionTM, el mayor repositorio de información científica curado por humanos, para comprender el panorama actual y los avances de la investigación en desextinción molecular. Hemos descubierto que los documentos han aumentado en los últimos diez años, con una patente durante ese tiempo (véase la figura 1).
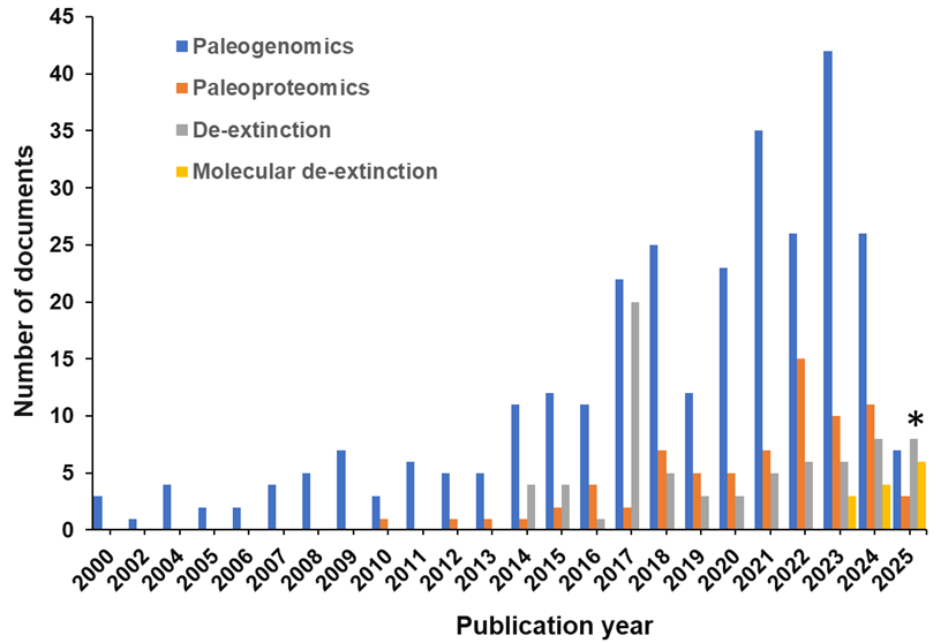
Este análisis revela que el pico de documentos relacionados con la desextinción en 2017 está relacionado con la publicación de un número especial de The Hastings Center for Bioethics, titulado Recreating the Wild: De‐Extinction, Technology, and the Ethics of Conservation. También examinamos los conceptos esenciales relacionados con la desextinción en la bibliografía (véase la figura 2).
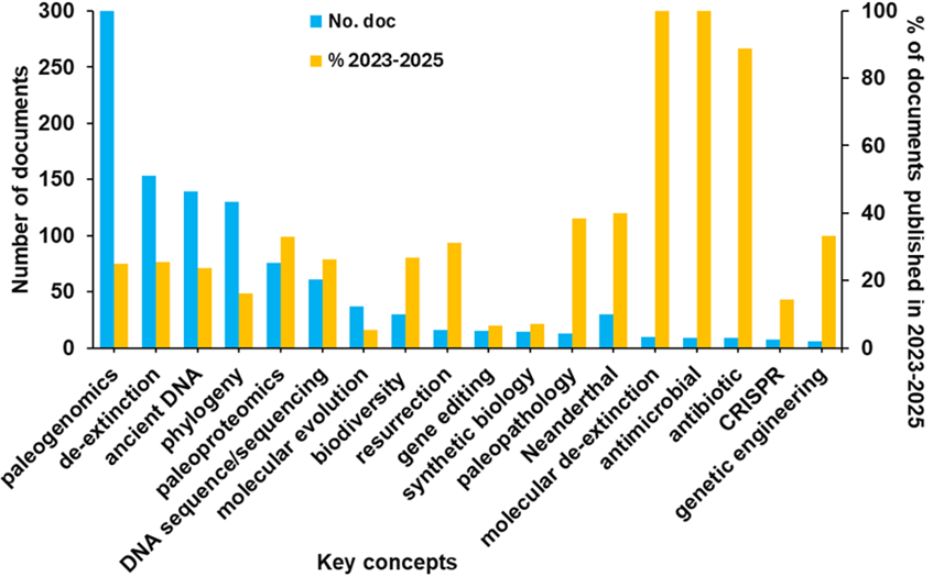
Las principales conclusiones del panorama actual de la investigación son:
- La paleogenómica es el concepto más explorado. Si bien otros enfoques (p. ej., la retrocruzamiento o la clonación) contribuyen a la desextinción, la paleogenómica proporciona la hoja de ruta genética necesaria para la recuperación precisa de especies. Mediante la recuperación, secuenciación y análisis del material genético de especies extintas, los investigadores pueden reconstruir genomas perdidos, identificar adaptaciones funcionales clave y diseñar sustitutos vivos mediante la edición del genoma. Los avances en la secuenciación de alto rendimiento, la edición genética CRISPR-Cas9 y la biología sintética están convergiendo para hacer de la recuperación de sustancias y especies un esfuerzo científico tangible, aunque complejo.
- Los antimicrobianos y los antibióticos son el tema de la mayoría de los documentos relacionados con la desextinción molecular en los últimos tres años (datos de 2025 solo hasta marzo). Cabe destacar tres intersecciones clave: 1) el descubrimiento de péptidos antimicrobianos antiguos a través de la paleogenómica, 2) las estrategias antimicrobianas basadas en CRISPR desarrolladas a partir de herramientas de desextinción y 3) el descubrimiento de nuevos antibióticos a partir de comunidades microbianas resucitadas.
- La paleopatología, el estudio de las enfermedades antiguas, coexiste con la desextinción. La paleopatología se centra tradicionalmente en comprender la salud de las poblaciones del pasado a través de restos óseos y momificados. Sin embargo, los recientes avances en paleogenómica, paleoproteómica y bioinformática han ampliado sus aplicaciones al descubrimiento de fármacos modernos. Mediante el análisis de patógenos antiguos, respuestas inmunitarias humanas y compuestos medicinales extintos, los investigadores están descubriendo nuevas estrategias terapéuticas para combatir la resistencia a los antibióticos, las enfermedades crónicas y las infecciones emergentes. Además, la integración de la biología evolutiva con la paleopatología ofrece una mejor comprensión de las presiones selectivas que afectaron a la susceptibilidad al cáncer en especies extintas y podría identificar posibles mecanismos de resistencia tumoral.
- Se han puesto a disposición datos a escala genómica sobre los neandertales a partir de los restos óseos de catorce yacimientos arqueológicos que abarcan la historia de los neandertales en gran parte de sus áreas geográficas identificadas.
Metodologías de la desextinción molecular
Como se ha señalado, existen dos enfoques para la desextinción: la paleogenómica, que estudia el ADN antiguo, y la paleoproteómica, que estudia las proteínas antiguas. Cada uno de ellos ha dado lugar a importantes avances en la identificación de posibles tratamientos antimicrobianos.
Paleogenómica
La idea de la desextinción ha pasado de ser ciencia ficción a convertirse en una búsqueda científica tangible gracias a los avances en paleogenómica. Este enfoque ya ha proporcionado conocimientos funcionales sobre la biología evolutiva, como los mecanismos de adaptación al frío de la megafauna del Pleistoceno, las diferencias neurogenéticas entre los humanos modernos y los neandertales, y la evolución del sistema inmunológico de los patógenos extintos.
La paleogenómica incluso nos ayuda a comprender su respuesta a los patógenos actuales. Por ejemplo, un estudio de los genes inmunitarios de los neandertales racionalizó su susceptibilidad a enfermedades infecciosas emergentes como la COVID-19. Un grupo de genes del cromosoma 3, identificado como el principal factor de riesgo genético de insuficiencia respiratoria tras la infección por SARS-CoV-2, fue conferido por un segmento genómico heredado de los neandertales y se ha descubierto que lo portan alrededor del 50 % de las personas del sur de Asia y el 16 % de las personas de Europa.
El proceso paleogénico tiene como objetivo revivir genes de especies extintas mediante la reconstrucción de sus genomas y su introducción en organismos vivos estrechamente relacionados, como en el ejemplo mencionado anteriormente del lobo gigante. El primer paso, y el más crucial, es obtener ADN antiguo de alta calidad a partir de material biológico conservado, seguido del aislamiento del ADN, la secuenciación de última generación y el ensamblaje genético computacional.
A diferencia del ADN moderno, el ADN antiguo está muy degradado, modificado químicamente y, a menudo, contaminado con ADN microbiano y ambiental. Los avances en la extracción de ADN, las tecnologías de secuenciación y la bioinformática han hecho posible recuperar y analizar el ADN antiguo, allanando el camino para los esfuerzos de desextinción.
Recuperar animales completamente formados, como lobos gigantes o mamuts lanudos, implica muchos retos prácticos y éticos. El bienestar animal y las consecuencias ecológicas son solo dos de las complejas cuestiones que rodean la desextinción de especies. Sin embargo, recuperar péptidos antiguos es mucho menos complicado y puede impulsar los esfuerzos de descubrimiento de fármacos. A través de la paleogenómica, los investigadores identificaron ocho genomas de vertebrados extintos y los verificaron computacionalmente en busca de defensinas, que son péptidos catiónicos pequeños y ricos en disulfuro que desempeñan un papel importante en la inmunidad del huésped.
Como resultado, se han identificado seis β-defensinas auténticas, cinco de las cuales proceden de dos especies diferentes de aves extintas y una de una especie de mamífero. Los análisis evolutivos y estructurales de estas moléculas están abriendo nuevas vías para el descubrimiento de antibióticos, aunque aún no se han validado experimentalmente.
Paleoproteómica
La desextinción molecular mediante paleoproteómica implica la extracción, secuenciación, reconstrucción computacional y resurrección funcional de proteínas de organismos extintos. Esta metodología aprovecha los avances en espectrometría de masas, bioinformática y biología sintética para recuperar y estudiar biomoléculas antiguas.
Por ejemplo, los científicos utilizaron modelos de aprendizaje profundo para descubrir nuevos péptidos antibióticos. Se entrenaron modelos para la predicción de sitios proteolíticos con el fin de proyectar la actividad antimicrobiana de una amplia gama de proteasas en los proteomas de organismos extintos (lo que se denomina «extinctoma»). Se predijo que una gran colección de secuencias no encontradas en organismos existentes exhibirían una actividad antimicrobiana de amplio espectro. De estas, se sintetizaron 69 péptidos y se verificó experimentalmente su actividad contra patógenos bacterianos. En la tabla 1 se muestra una selección de péptidos antimicrobianos activos.
| Péptido | Organismo extinto | Proteína parental | Secuencia peptídica | MIC |
|---|---|---|---|---|
| Hydrodamin-1 3078251-51-8 |
Vaca marina de Steller (Hydrodamalis gigas) | Gen de diferenciación endotelial 1 | LYCRIYSLVRARG RRLTFRKNISK | 4 μmol L-1 (A. baumannii, E. faecium) |
| Megalocerin-1 3078251-56-3 |
Alce gigante (Megaloceros gigantescus) | Subunidad 3 de la citocromo c oxidasa | LIVCFFRQLKFHF | 8 μmol L-1 (A. baumannii, E. faecium) |
| Mylodonin-2 3078251-68-7 |
Perezoso gigante (Mylodon darwinii) | Apolipoproteína B | KRKRGLKLATALS LNNKF | 32 μmol L-1 (E. coli) |
| Elephasin-2 3078251-59-6 |
Elefante de colmillos rectos (Elephas antiquus) | Subunidad 8 de la ATP sintasa F0 | IFLHLKILKIIRLL | 1 μmol L-1 (A. baumannii, S. aureus, E. faecium) |
| Mammuthusin-2 3078251-49-4 |
Mamut lanudo siberiano (Mammuthus primigenius) | Receptor de melanocortina-1 | RACLHARSIARLHK RWRPVHQGLGLK | 32 μmol L-1 (A. baumannii, E. faecium) |
| Equusin-1 3078251-35-8 |
Cebra de Grant (Equus quagga boehmi) | Proteína 1 de los macrófagos asociada a la resistencia natural | FLKLRWSRFARVLL | 1 μmol L-1 (E. faecium) 4 μmol L-1 (A. baumannii, E. coli, P. aeruginosa) |
| Equusin-2 3078251-48-3 |
Cebra de Grant (Equus quagga boehmi) | Proteína asociada a la microcefalia anómala en forma de huso | KIYKKLSTPPFTL NIRTLPKVKFPK | 8 μmol L-1 (A. baumannii) |
Varios pares de péptidos del mismo organismo extinto mostraron fuertes interacciones sinérgicas contra patógenos como A. baumannii y P. aeruginosa, con valores de índice de concentración inhibitoria fraccional (FIC) tan bajos como 0,38 para A. baumannii. En el caso de la combinación de Equusin-1 y Equusin-3, las concentraciones inhibitorias mínimas (MIC) disminuyeron 64 veces (de 4 μmol L-1 a 62,5 nmol L-1), alcanzando concentraciones submicromolares comparables a las MIC de los antibióticos más potentes.
Cabe destacar que los compuestos más destacados, entre los que se incluyen Mammuthusin-2, Elephasin-2, Hydrodamin-1, Mylodonin-2 y Megalocerin-1, mostraron una actividad antiinfecciosa potencial en ratones con abscesos cutáneos o infecciones en los muslos. Los resultados obtenidos para los péptidos más activos probados en el modelo de infección por absceso cutáneo (Elephasin-2 y Mylodonin-2) indicaron una actividad antibacteriana comparable a la del antibiótico polimixina B, ampliamente utilizado. Del mismo modo, Mylodonin-2 y Elephasin-2 mostraron una eficacia antiinfecciosa comparable a la de la polimixina B cuando se utilizó un modelo murino de infección profunda en el muslo, lo que subraya el potencial de la desextinción molecular como un enfoque exitoso para el descubrimiento de antibióticos.
Una patente reciente reveló los métodos para identificar péptidos antimicrobianos derivados de proteomas extintos utilizando un algoritmo de aprendizaje profundo multitarea, APEX, junto con los 41 péptidos antimicrobianos identificados y su sinergia y mecanismo de acción.
Además, la herramienta de aprendizaje automático creada, el modelo de bosque aleatorio panCleave para la predicción de sitios de escisión en todo elproteoma, exploró un clasificador de sitios de escisión de panproteasas para realizar la proteólisis computacional, una digestión in silico de proteínas humanas. Por lo tanto, se utilizaron enfoques de aprendizaje automático para la desextinción molecular y se extrajeron los proteomas de nuestros parientes más cercanos, los humanos arcaicos neandertales y denisovanos. Se resucitaron varios antibióticos peptídicos encriptados, que mostraron actividad antimicrobiana in vitro y en modelos preclínicos con ratones.
Posibles avances en antibióticos
Para comprender cómo podrían haber sido los antepasados de los actuales antibióticos glucopéptidos, los investigadores utilizaron bioinformática y métodos genéticos y bioquímicos para devolver a la vida la «paleomicina» ancestral. En primer lugar, se predijo la cadena de montaje de la sintetasa peptídica no ribosómica de la paleomicina y se construyó un árbol guía basado en grupos de genes biosintéticos. Posteriormente, mediante técnicas de biología sintética, los investigadores reconstruyeron el péptido predicho y validaron su actividad antibiótica.
Este estudio demostró que la combinación de la biología sintética y las técnicas computacionales puede determinar la evolución temporal de los antibióticos y potencialmente revivir moléculas antiguas. También demostró las tácticas de optimización natural logradas a través de procesos evolutivos que dieron lugar a los antibióticos glucopéptidos modernos, sentando así las bases para futuros esfuerzos para diseñar esta importante clase de agentes antimicrobianos.
Los científicos también están explorando la posibilidad de encontrar nuevas fuentes de antibióticos en las catelicidinas neandertales. Se trata de una familia de péptidos antimicrobianos similares a los que se encuentran en los humanos modernos, que desempeñan un papel en la defensa contra las infecciones. Los investigadores han desarrollado un modelo de aprendizaje automático que puede extraer datos proteómicos y genómicos de los neandertales y los denisovanos, esencialmente encontrando secuencias de humanos arcaicos y prediciendo cuáles serían candidatos viables para antibióticos.
Retos y oportunidades de la desextinción
La desextinción molecular puede presentar menos dilemas logísticos y éticos que resucitar animales extintos y reintroducirlos en los ecosistemas modernos. Sin embargo, no está exenta de ciertos riesgos, como p. ej.:
- La degradación del ADN y los datos genómicos incompletos dificultan la reconstrucción completa de los genes.
- La incertidumbre funcional de las moléculas resucitadas, incluidos los errores de plegamiento de proteínas, las modificaciones postraduccionales, la toxicidad y la inmunogenia.
- El silenciamiento génico, los efectos fuera del objetivo y la transferencia génica horizontal, en la que los genes modificados podrían propagarse de forma incontrolada en los ecosistemas y tener repercusiones no deseadas.
- Cuestiones bioéticas sobre si las moléculas extintas deben comercializarse.
Los marcos éticos y la colaboración entre las comunidades científica y reguladora serán fundamentales para orientar muchas de estas consideraciones. En cuanto a los posibles obstáculos científicos, las tecnologías avanzadas seguirán desempeñando un papel clave. La IA puede simular cómo se pliegan y funcionan las proteínas, evitando la necesidad de secuencias completas de ADN. Las redes neuronales pueden predecir los fragmentos que faltan en el ADN antiguo degradado y mejorar la precisión de la reconstrucción. CRISPR-Cas9 y la edición de bases pueden «humanizar» potencialmente los genes antiguos para un uso médico seguro.
La desextinción molecular representa un cambio de paradigma en el descubrimiento de antibióticos, ya que ofrece una reserva única de potencial antimicrobiano sin explotar. Aunque siguen existiendo retos en cuanto a la escalabilidad y la regulación, los primeros éxitos demuestran que la biodiversidad perdida de la Tierra puede ser la clave para resolver la crisis de la resistencia a los antimicrobianos.
Para obtener más información, consulte nuestro artículo en ACS Omega, Molecular Paleontology Meets Drug Discovery: The Case for De-extinct Antimicrobials.
Referencias:
2: https://www.nature.com/articles/s41551-024-01201-x?utm_source=acs&getft_integrator=acs







