As substâncias per e polifluoroalquil, comumente conhecidas como PFAS, representam uma classe de moléculas orgânicas altamente fluoradas produzidas pelo homem, caracterizadas por ligações carbono-flúor excepcionalmente fortes. Essas ligações criam materiais com propriedades notáveis: resistência à degradação química e física, repelência à água e ao óleo, capacidade de emulsificação e estabilidade em altas temperaturas.
Essas características tornaram os PFAS valiosos em diversos setores, incluindo produtos domésticos comuns (panelas antiaderentes, itens de higiene pessoal etc.), produtos farmacêuticos, plásticos, eletrônicos, combate a incêndios, cosméticos, automotivo, aeroespacial e armazenamento de energia.
{{pfas="/ads"}}
Entretanto, essas mesmas propriedades — e a força das ligações carbono-flúor — levantaram preocupações sobre a persistência ambiental dos PFAS e os possíveis impactos na saúde humana. Consequentemente, os órgãos regulatórios no mundo todo estão respondendo com regras e restrições em constante evolução e os consumidores estão cada vez mais pedindo alternativas.
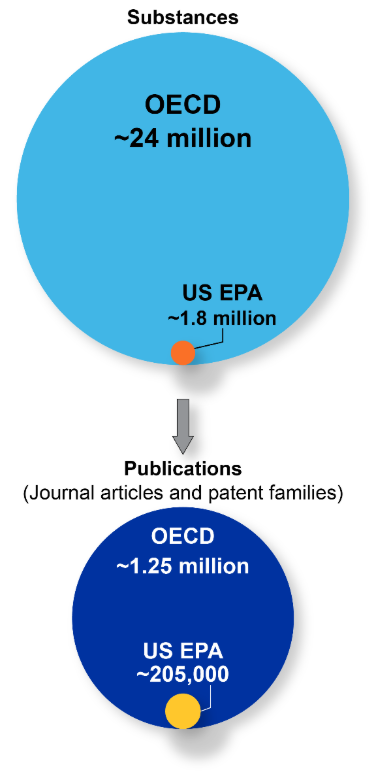
Para estabelecer uma compreensão mais profunda da prevalência de PFAS em publicações científicas, fizemos buscas sistemáticas baseadas na estrutura química dentro da Coleção de conteúdo do CASTM, o maior repositório de informações científicas curado por humanos. Nossa análise revelou diferenças marcantes na identificação de PFAS com base em definições regulatórias variadas (ver a Figura 1):
- Seguindo as diretrizes de definição da Organização para Cooperação e Desenvolvimento Econômico (OCDE) para 2021 (que incluem compostos que contêm até mesmo um único grupo -CF₃ ou -CF₂), aproximadamente 24 milhões de moléculas distintas de PFAS foram identificadas na Coleção de Conteúdo do CAS.
- Em contrapartida, com a aplicação da definição mais rigorosa da Agência de Proteção Ambiental dos Estados Unidos (U.S. EPA) (que exige pelo menos dois grupos -CF₂ ou -CF₃), o número de substâncias classificadas como PFAS diminuiu consideravelmente para aproximadamente 1,8 milhão de compostos.
Esses números são consideravelmente mais altos do que os 10 mil a 15 mil compostos de PFAS estimados, e isso significa que muitos mais produtos podem ser afetados pelas regulamentações ou precisar de alterações na formulação para alcançarem propriedades semelhantes em suas aplicações. Concentrando-nos em substâncias com relevância científica ou comercial estabelecida, analisamos mais de 350 mil compostos únicos que aparecem em mais de um milhão de documentos científicos.
Apesar de um aumento distinto nas publicações sobre mitigação ou remediação de PFAS ao longo do tempo (ver Figura 2), o volume atual da pesquisa ainda é insuficiente, dado o uso generalizado e o impacto ambiental dos PFAS. É importante notar que os EUA se destacam como os mais influentes e proativos em termos de pesquisa de mitigação de PFAS, impulsionando avanços significativos neste campo. A UE e a China também emergem como atores-chave, intensificando as iniciativas de pesquisa para enfrentar os desafios relacionados aos PFAS.
Isso ainda destaca a necessidade de intensificar os esforços globais para desenvolver métodos eficazes para mitigar a contaminação por PFAS, garantindo assim a segurança ambiental e protegendo a saúde pública. Nossa análise abrange o cenário regulatório em evolução e a prevalência de PFAS em 25 aplicações principais. Identificamos tendências de publicação, bem como possíveis alternativas em várias aplicações, com base em documentos da Coleção de Conteúdo do CAS.
Mudanças geográficas e padrões comerciais na literatura sobre PFAS
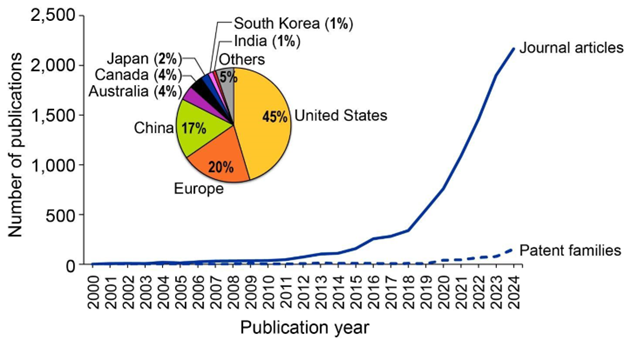
Em nossa análise, examinamos 1 milhão de documentos da Coleção de Conteúdo do CAS que faziam referência a mais de 350 mil compostos PFAS exclusivos. A análise temporal revela uma tendência ascendente significativa no número de publicações de periódicos e patentes nos últimos 70 anos (ver Figura 3). Apesar das rigorosas regulamentações globais, a proporção de patentes para periódicos de 2:3 indica que os compostos PFAS continuam sendo amplamente utilizados em várias aplicações industriais.

Nossa análise da distribuição global dessas publicações revela que, embora os EUA continuem proeminentes, os países asiáticos, particularmente China e Japão, também são centros importantes para pesquisa e aplicação de PFAS (ver Figura 4). Essa tendência pode ter como resultados a ausência de regulamentações rigorosas de PFAS nessas regiões. Além disso, os países europeus apresentam números baixos de publicação de patentes, sugerindo uma tendência de queda ou redução do uso de PFAS nos setores, provavelmente relacionada a uma possível proibição seletiva de PFAS na UE.
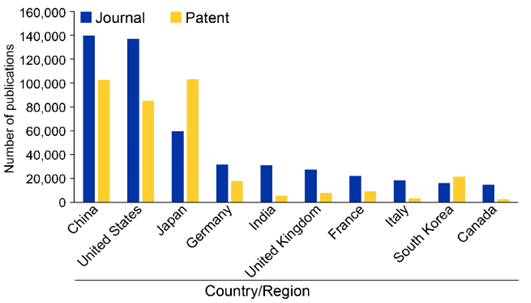
O uso comercial de PFAS em vários países foi avaliado analisando o número de patentes publicadas por vários setores nessas regiões (ver Figura 5). Notavelmente, o Japão está na vanguarda do uso de PFAS para aplicações comerciais, seguido pelos EUA e China. A análise temporal na Figura 5 das publicações de patentes por esses países revela que a China é o único país que está experimentando um aumento significativo em patentes comerciais relacionadas a PFAS, enquanto o uso comercial diminuiu em todos os outros países, exceto na Coreia do Sul.

Como veremos, esses padrões refletem o uso generalizado de PFAS em vários setores e aplicações e a diversidade de abordagens regulatórias em todas as regiões geográficas.
O cenário regulatório em mudança dos PFAS
A regulamentação dos PFAS apresenta desafios complexos que vão além da simples eliminação, exigindo um equilíbrio delicado entre a redução dos riscos ambientais e à saúde e a manutenção das funções essenciais. O cenário regulatório é complicado pela diversidade dos compostos de PFAS; pela incerteza científica em relação à sua toxicidade, persistência e bioacumulação; e pelo papel crítico desses químicos em setores como saúde, defesa e segurança contra incêndios.
Além disso, há barreiras práticas no processo de remoção ou substituição de PFAS, incluindo limitações de detecção, custos de remediação e falta de alternativas adequadas para algumas aplicações críticas. Isso exige abordagens regulatórias diferenciadas que priorizem a eliminação gradual de usos não essenciais, enquanto gerenciam transições cuidadosas para aplicações onde a eliminação imediata criaria interrupções inaceitáveis ou preocupações com a segurança.
Diferentes áreas geográficas tomaram medidas para regulamentar os PFAS nos últimos 15 anos, com o aumento da atividade nos últimos cinco anos, à medida que aumentam as preocupações públicas sobre os efeitos dos PFAS no ambiente e na saúde humana (ver Figura 6).

Regulamentações dos EUA
A EPA dos EUA implementou medidas para restringir ou banir seletivamente os PFAS ao longo do tempo. Em 2023, a agência introduziu uma nova estrutura para estabelecer um processo de revisão mais rigoroso: PFAS com exposição insignificante e risco de liberação ambiental podem ser comercializados após receberem dados básicos de propriedades físico-químicas e garantirem que os PFAS possam ser descartados corretamente. PFAS com potencial de liberação baixo, mas não desprezível, exigem testes adicionais, como dados toxicocinéticos, antes da aprovação da fabricação.
Para PFAS com probabilidade de causar lançamentos ou exposições ambientais significativas (como protetores de manchas aplicados por spray), a EPA normalmente proíbe a comercialização até que sejam concluídos testes extensivos sobre propriedades físicas/químicas, toxicidade e destino ambiental, a menos que haja uma necessidade militar crítica.
O Roteiro Estratégico de PFAS de 2024 da EPA designou os dois PFAS mais amplamente usados e prejudiciais, o PFOA e o PFOS, bem como seus sais e isômeros estruturais, como substâncias perigosas de acordo com a Lei de Resposta, Compensação e Responsabilidade Ambiental Abrangente (CERCLA, comumente conhecida como Superfund). Essa designação levou a vários requisitos de relatórios e obrigações de divulgação para o setor, proprietários de imóveis e órgãos federais, permitindo que a EPA abordasse mais locais contaminados, tomasse medidas antecipadas e agilizasse eventuais limpezas.
Há também muitas regulamentações em nível estadual que frequentemente exigem notificações de produtos, certificações de conformidade para fabricantes e distribuidores, e proibições totais de certas categorias de produtos com PFAS adicionado intencionalmente. Califórnia, Maine, Washington e outros implementaram proibições abrangentes de PFAS em várias categorias de produtos de consumo.
As empresas podem ter dificuldades com as diversas regulamentações estaduais, mas essas ações estão afastando os mercados dos químicos PFAS, apesar da falta de leis federais fortes. À medida que a preocupação pública com esses "químicos eternos" persistentes aumenta, mais estados estão abordando proativamente a questão em vez de esperar pela intervenção federal.
Regulamentações da UE sobre PFAS
A União Europeia (UE) geralmente tem restrições mais rigorosas sobre PFAS do que os EUA sob a Convenção de Estocolmo. Os estados-membros também implementaram proibições parciais, como na Dinamarca, onde a importação e venda de roupas, calçados e seus agentes impermeabilizantes contendo PFAS serão proibidas na maioria dos casos a partir de julho de 2026.
A Agência Europeia de Produtos Químicos (ECHA) estima que, se as práticas atuais continuarem de forma semelhante, 4,4 milhões de toneladas métricas de PFAS serão despejadas no ambiente nas próximas três décadas. Esse cenário apresenta riscos substanciais ao ambiente e à saúde pública devido à natureza persistente das emissões de PFAS. Consequentemente, em 2023, a ECHA colaborou com as autoridades europeias da Dinamarca, Alemanha, Países Baixos, Noruega e Suécia para apresentar uma proposta que proibiria efetivamente todos os PFAS que se encaixam na definição da OCDE (com algumas isenções) na Europa.
As agências da UE receberam milhares de comentários sobre a legislação proposta, incluindo preocupações sobre possíveis perdas de empregos e interrupções na cadeia de suprimento. No momento em que este texto foi escrito, continuam as deliberações sobre uma possível proibição e quaisquer implicações econômicas.
Regulamentações da Ásia-Pacífico
A Convenção de Estocolmo influencia grande parte das regulamentações de PFAS na região Ásia-Pacífico. Países como China, Japão, Coreia do Sul, Austrália e Nova Zelândia proibiram ou restringiram as substâncias PFAS. Entretanto, o cenário regulatório nesses países continua evoluindo, com vários deles adotando medidas progressivas que espelham as abordagens adotadas nos EUA e na UE.
Padrões de uso de PFAS por aplicação
Visão geral
Em nossa análise da Coleção de Conteúdo do CAS, classificamos as publicações em várias aplicações com base nas seções temáticas do CAS. Reduzimos nosso foco para 25 aplicações principais com base em sua alta proporção de patentes por periódico (veja Figura 7).
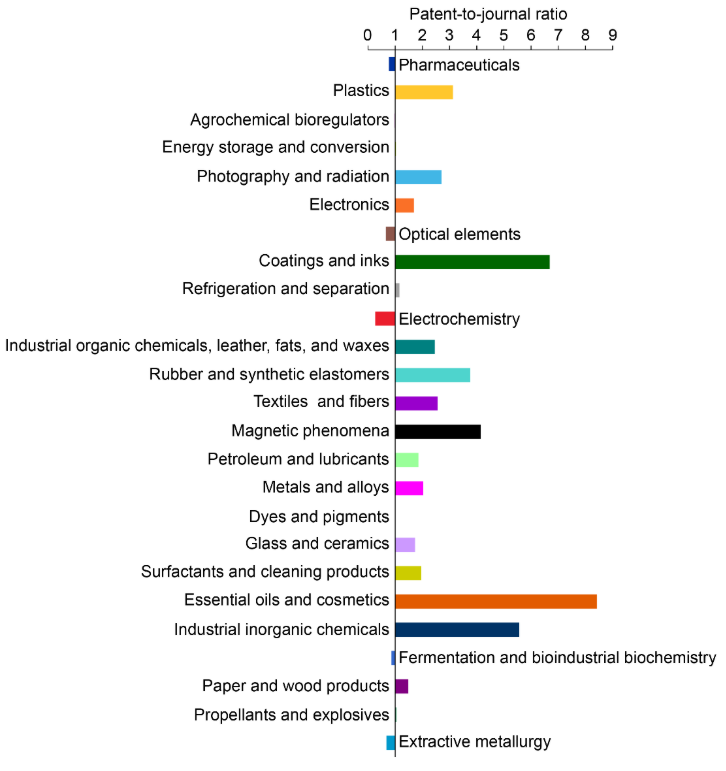
A partir daí, conduzimos uma análise detalhada das relações entre substâncias químicas e conceitos científicos em 10 aplicações selecionadas, escolhidas com base em seus perfis distintos de conteúdo de flúor e maiores proporções de patentes/publicações em periódicos.
Plásticos
PFAS são incorporados em várias formulações de plástico para conferir propriedades críticas de desempenho, incluindo resistência química aprimorada, estabilidade térmica e redução de atrito. Como se vê na Figura 8, pequenas moléculas e polímeros são as classes predominantes de substâncias PFAS.
Nos plásticos convencionais, os PFAS funcionam como agentes desmoldantes e aditivos antibloqueio que impedem a adesão entre as superfícies plásticas durante a fabricação. Os PFAS têm sido usados extensivamente em embalagens plásticas de alimentos, onde se descobriu que migram para alimentos, levantando preocupações sobre a exposição humana. Já há inúmeras alternativas para embalagens de alimentos, como polietileno e resinas e óleos de silicone.

Para descobrir as funções das moléculas de PFAS usadas com frequência em plásticos, analisamos a coocorrência de conceitos científicos importantes e substâncias PFAS. Como mostra a Figura 8, algumas moléculas de PFAS, como 1107-00-2 (4,4-(hexafluoro-isopropilideno)anidrido diftálico) e 341-58-2 (2,2'-bis(trifluorometil)-4,4'-diaminobifenil), aparecem com destaque em publicações que discutem filmes plásticos e dispositivos ópticos de imagem. Outros, como 9011-17-0 (copolímero de fluoreto de hexafluoropropileno-vinilideno) e 25067-11-2 (copolímero de hexafluoropropileno-tetrafluoroetileno), aparecem em vários conceitos de aplicação como incorporação de negro de fumo, filmes plásticos, materiais de revestimento e membranas.
Outras aplicações
Em nossa análise da literatura relacionada aos PFAS na Coleção de conteúdo do CAS, examinamos várias aplicações industriais, incluindo biorreguladores agroquímicos; armazenamento e conversão de energia; fotografia e radiação; eletrônica, especificamente o uso de PFAS na fabricação de semicondutores e tecnologias de bateria; revestimentos e tintas; borracha e elastômeros sintéticos; e substâncias de refrigeração e separação. As moléculas pequenas são uma das principais classes de substâncias nessas aplicações, assim com polímeros e sais. Analisamos também as substâncias PFAS nas categorias de consumo óleos essenciais e cosméticos e têxteis e tecidos. Os PFAS são amplamente utilizados nessas aplicações, mas essas categorias também têm os materiais de substituição mais viáveis.
É importante observar que os produtos farmacêuticos são numericamente a aplicação mais prevalente na literatura. Encontramos quase 560 mil documentos que incluem uma referência a PFAS relacionada a vários medicamentos, transportadores de medicamentos, sistemas de liberação de medicamentos e o estudo de suas funções. Entretanto, as moléculas de PFAS em aplicações farmacêuticas geralmente apresentam estruturas químicas diferentes das outras moléculas de PFAS de cadeia longa, embora tecnicamente atendam à definição de PFAS da OCDE. Os produtos farmacêuticos também estão sendo classificados como "uso essencial" das moléculas de PFAS devido ao seu papel fundamental nos medicamentos e à atual falta de alternativas não fluoradas.
Para uma discussão detalhada sobre essas aplicações, baixe o relatório completo.
Desafios na eliminação gradual dos PFAS
Nosso relatório completo apresenta uma análise sem precedentes de PFAS, oferecendo uma perspectiva baseada em dados que transforma nossa compreensão desta classe química crítica. Nossa análise abrangente da Coleção de conteúdo do CAS revela que o universo dos PFAS é substancialmente maior e mais estruturalmente diverso do que comumente entendido no discurso regulatório e científico, com implicações significativas para o desenvolvimento de políticas e planejamento de transição.
As relações entre estrutura e função reveladas em nossa análise demonstram por que os PFAS se tornaram tão profundamente integrados em todos os setores — suas propriedades exclusivas são deliberadamente projetadas por meio de padrões específicos de flúor adaptados aos requisitos da aplicação. Isso explica seu valor técnico e o desafio de encontrar alternativas adequadas.
Nossa análise Global regulatória revela um cenário fragmentado evoluindo em diferentes ritmos e com abordagens distintas. Isso cria um ambiente de conformidade complexo para operações multinacionais e sugere que as mudanças regionais na fabricação e inovação são prováveis à medida que as empresas se adaptam a requisitos variados. O contraste entre a abordagem abrangente da UE, por exemplo, e a estratégia federal mais seletiva dos EUA destaca a tensão fundamental entre os princípios de precaução e a regulamentação baseada em riscos na abordagem de químicos de preocupação emergente.
O Futuro cenário dos PFAS será moldado pela interação entre inovação técnica, evolução regulatório e transformação do mercado. As organizações que desenvolverem estratégias abrangentes e específicas para cada aplicação, com base no entendimento estrutural de seu portfólio de PFAS, estarão mais bem posicionadas para navegar nesta complexa transição. Aqueles que se envolvem proativamente em discussões regulatórias com avaliações baseadas em evidências da viabilidade técnica ajudarão a moldar abordagens de política mais eficazes que protejam o ambiente e a saúde pública, preservando tecnologias essenciais.