Nos últimos anos, tem havido uma ênfase crescente no microbioma intestinal e seu papel na saúde humana em geral. De forma relacionada, a saúde animal vem sendo cada vez mais compreendida sob a ótica desse complexo ecossistema de microrganismos que desempenha um papel fundamental na digestão, na imunidade e na resistência a doenças. As pessoas estão preocupadas com o uso de antibióticos em animais de produção que se tornam fontes de alimentos, assim como com o bem-estar animal e a saúde de nossos animais de estimação, todos aspectos relacionados ao microbioma intestinal.
Como resultado, pesquisadores estão explorando formas de proteger e aprimorar a saúde animal por meio de estratégias nutricionais e terapêuticas, algumas semelhantes ao mercado de soluções para o microbioma humano e outras inéditas no reino animal.
O alicerce da saúde animal
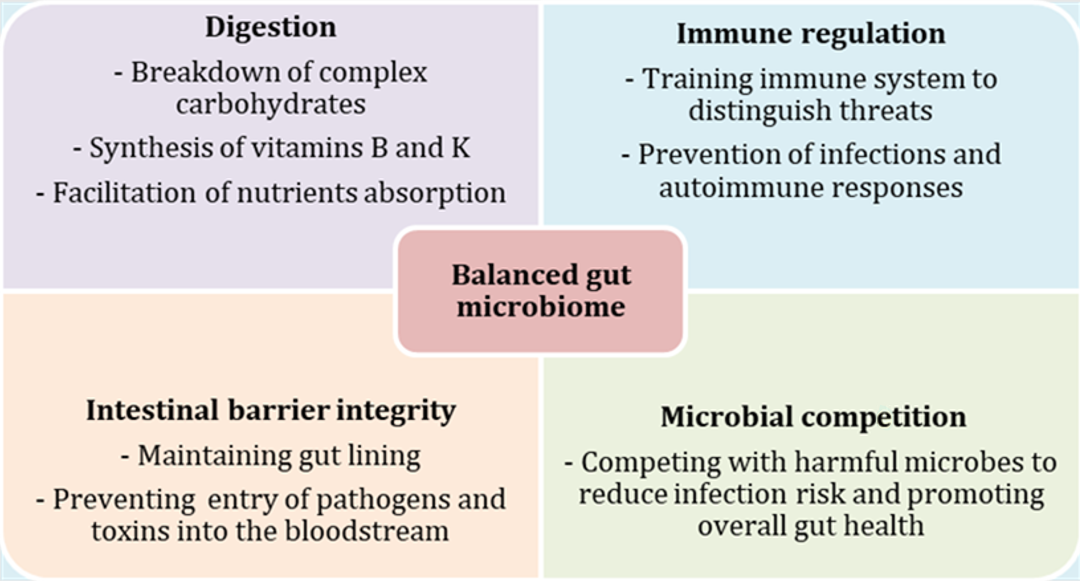
O microbioma intestinal é composto por trilhões de microrganismos, incluindo bactérias, arqueias, vírus e fungos, que residem no trato digestivo. Esses microrganismos desempenham papéis essenciais na digestão de nutrientes, no desenvolvimento do sistema imunológico e na proteção contra patógenos. Um microbioma equilibrado favorece a eficiência alimentar, o crescimento e a resiliência em animais de produção e de companhia (ver Figura 1). Desequilíbrios no balanço microbiano, conhecidos como disbiose, estão associados a distúrbios gastrointestinais, doenças metabólicas e redução da produtividade.
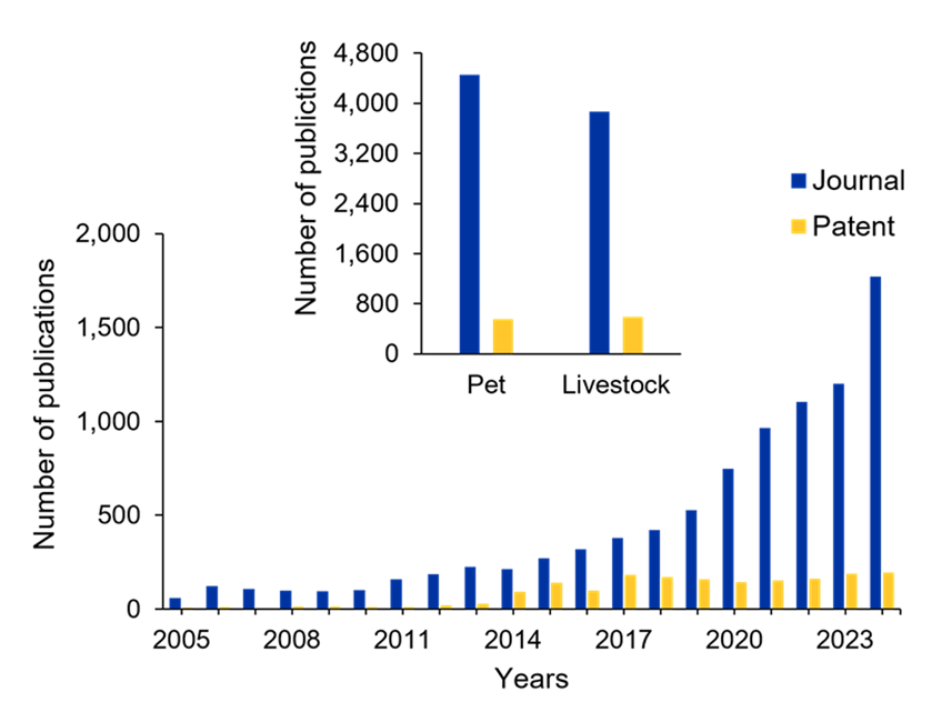
Analisamos a Coleção de conteúdo do CASTM, o maior repositório de informações científicas curadas por humanos, e identificamos uma evolução notável no cenário de pesquisas relacionadas ao microbioma intestinal animal. De 2005 a 2024, houve uma tendência consistente de crescimento nas publicações em periódicos nesse campo (ver Figura 2). A comparação do quadro interno mostra que os animais de estimação apresentam ligeiramente mais publicações no total do que os animais de produção, e que os artigos de periódicos superam de forma consistente as patentes em ambas as categorias.
Isso demonstra o maior foco na saúde de animais de estimação nos últimos anos e que a maior parte das pesquisas nesse campo ainda não chegou à comercialização. No entanto, diversas estratégias terapêuticas vêm ganhando destaque com base em nossa análise. Como vemos na Figura 3A, muitas doenças estão associadas à disbiose do microbioma intestinal, sendo as condições gastrointestinais, metabólicas e relacionadas ao sistema imunológico as mais documentadas. A Figura 3B destaca estratégias terapêuticas voltadas à restauração do equilíbrio microbiano, com probióticos e prebióticos recebendo a maior atenção em pesquisas. Essa comparação ressalta o crescente interesse científico em intervenções direcionadas ao microbioma para diversas condições de saúde.
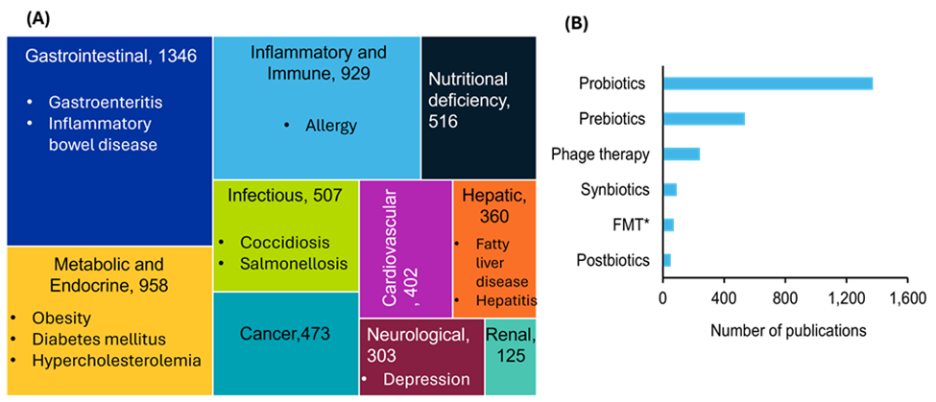
Por exemplo, em animais de produção como bovinos, aves, suínos e pequenos ruminantes, a saúde intestinal está intimamente ligada à eficiência alimentar, ao ganho de peso, ao sucesso reprodutivo e à resistência a doenças. Em animais de companhia, como cães e gatos, o microbioma afeta não apenas a digestão e a imunidade, mas também o humor, o comportamento, a condição da pele e a vitalidade geral. A disbiose em animais de estimação tem sido associada a condições crônicas, incluindo obesidade, alergias, doença inflamatória intestinal, câncer intestinal e ansiedade, evidenciando o amplo impacto do microbioma na nutrição animal e na qualidade de vida.
Abordagens terapêuticas atuais para modular a microbiota intestinal
Quando o microbioma intestinal se torna desequilibrado devido a fatores como uso de antibióticos, dieta inadequada, estresse ou infecções, isso pode levar à disbiose, que se manifesta como distúrbios gastrointestinais, inflamação, problemas metabólicos e imunidade enfraquecida. Diversas estratégias terapêuticas surgiram para tratar a disbiose:
Probióticos e prebióticos
Os probióticos — microrganismos benéficos vivos, como lactobacillus, bifidobacterium e enterococcus — desempenham um papel vital na melhoria da saúde intestinal dos animais. Eles ajudam a restaurar o equilíbrio microbiano, suprimir patógenos, melhorar a absorção de nutrientes e reduzir distúrbios gastrointestinais, especialmente durante períodos de estresse, transições alimentares e no desenvolvimento inicial. Estudos em bezerros, leitões e iaques mostraram que dietas ricas em probióticos melhoram a morfologia intestinal, a diversidade microbiana, as respostas imunológicas e o desempenho de crescimento, além de reduzir a inflamação e favorecer a digestão. Essas evidências demonstram o potencial dos probióticos e simbióticos para promover a produção pecuária sem antibióticos e otimizar a microbiota intestinal para a saúde animal de forma geral.
Os prebióticos, como frutooligossacarídeos (FOS), inulina e manano-oligossacarídeos (MOS), são fibras alimentares não digeríveis que estimulam seletivamente o crescimento de bactérias intestinais benéficas. A fermentação desses compostos pelos microrganismos intestinais leva à produção de ácidos graxos de cadeia curta (AGCC), especialmente butirato, acetato e propionato, que desempenham um papel fundamental na manutenção da integridade da barreira intestinal, na redução da inflamação e no suporte à saúde metabólica.
Pós-bióticos
Os pós-bióticos, que são metabólitos ou componentes estruturais produzidos por probióticos, como ácido lático e peptídeos, oferecem uma alternativa estável e não viva para aprimorar a saúde animal. Quando adicionados à ração, eles podem modular as respostas imunológicas mucosas e sistêmicas, apoiando a integridade intestinal e reduzindo a inflamação. Um ensaio clínico recente da Admixture Research Company demonstrou o potencial dos pós-bióticos na saúde de animais de companhia. Em cães adultos, a forma pós-biótica tratada termicamente de Bifidobacterium animalis subsp. lactis CECT 8145 (PRIOME® MH) reduziu de forma significativa os níveis de glicose no sangue pós-prandial durante uma fase de perda de peso, sugerindo seu papel no suporte à saúde metabólica.
Transplante de microbiota fecal
O transplante de microbiota fecal (TMF) vem ganhando reconhecimento como uma valiosa ferramenta terapêutica na medicina veterinária, com aplicações em animais de companhia e de produção. Em cães e gatos, a FMT tem mostrado potencial no tratamento de distúrbios gastrointestinais como diarreia associada a antibióticos, diarreia crônica e doença inflamatória intestinal (DII), melhorando significativamente os sintomas clínicos e restaurando o equilíbrio microbiano (Ref). Na pecuária, o FMT tem sido explorado em ruminantes como bezerros e vacas leiteiras para Gerenciar a diarréia e até mesmo melhorar a saúde do úbere e a qualidade do leite em casos de mastite subclínica. Em suínos, particularmente em porcos jovens, o FMT tem sido eficaz na redução da diarreia pós-desmame, aumentando o ganho de peso e melhorando a composição da microbiota intestinal. Essas aplicações destacam o potencial do TMF para melhorar a saúde, a produtividade e o bem-estar animal.
Terapia com fagos
A terapia com bacteriófagos é uma alternativa emergente aos antibióticos no tratamento de infecções bacterianas devido às suas múltiplas vantagens como medicamentos adaptativos. Entre essas vantagens estão sua alta especificidade e sua capacidade de evoluir e se multiplicar no local das infecções. Entre as aplicações promissoras estão a prevenção da mastite em bovinos causada por Staphylococcus aureus, a mitigação da salmonelose em suínos devido à Salmonella enteritidis e o controle de Campylobacter jejuni e Salmonella Gallinarum em aves. Avanços recentes na engenharia genética, particularmente a integração dos sistemas CRISPR/Cas9, aumentaram ainda mais a precisão e a eficácia da terapia com fagos.
Terapêutica de próxima geração: combatendo a resistência antimicrobiana
Como observado nas abordagens terapêuticas atuais, como pré e probióticos, é fundamental abordar o uso de antibióticos e a resistência a antibióticos associada. A resistência antimicrobiana (RAM) impacta a segurança alimentar, a saúde humana e a medicina veterinária, pois o microbioma intestinal atua como um importante reservatório de genes de resistência antimicrobiana (ARGs). O uso indevido disseminado de antimicrobianos em animais de produção e de companhia acelerou a resistência, sendo que a Ásia respondeu por 67% das cerca de 99.500 toneladas de uso global de antimicrobianos veterinários em 2020. Esse número deverá crescer 8% até 2030, ressaltando a necessidade de intervenções urgentes e coordenadas que considerem a complexa interação entre o uso de antimicrobianos, a disrupção do microbioma intestinal e o desenvolvimento da resistência.
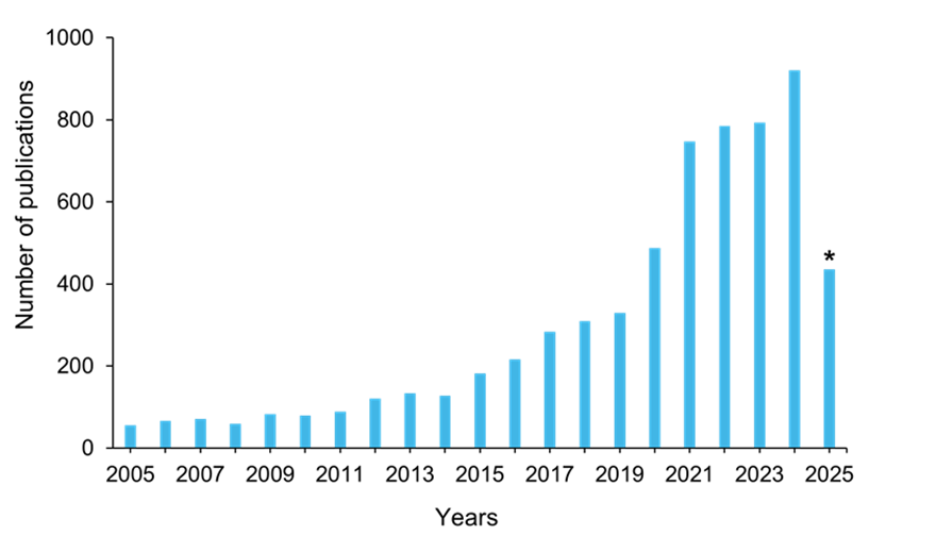
Os esforços de pesquisa se intensificaram em resposta a esse cenário, como refletido no crescimento constante das publicações relacionadas à RAM na CAS Content Collection ao longo das últimas duas décadas (ver Figura 4). Diversas estratégias inovadoras estão atualmente em desenvolvimento.
Intervenções focadas na sustentabilidade
Tratamentos focados no microbioma podem ajudar a melhorar simultaneamente a saúde animal e a sustentabilidade ambiental. Um estudo demonstrou que determinadas bactérias intestinais conseguem absorver e eliminar substâncias perfluoroalquil e polifluoroalquil (PFAS), uma classe de poluentes ambientais persistentes. Em ratos, esses microrganismos reduziram os níveis sistêmicos de PFAS em até 75%, sugerindo um caminho promissor para estratégias de desintoxicação em animais.
Outro avanço promissor é o uso da tecnologia CRISPR para projetar probióticos capazes de reduzir as emissões de metano em bovinos. Como o metano, um potente gás de efeito estufa, é produzido principalmente por arqueias metanogênicas no rúmen, modificar a composição microbiana no início da vida pode reduzir significativamente as emissões sem afetar a produtividade, oferecendo uma Solução sustentável para o setor pecuário. Essa intervenção baseada no microbioma oferece uma estratégia sustentável para a indústria pecuária, especialmente considerando que as emissões do setor representam aproximadamente 14,5% das emissões globais de gases de efeito estufa.
Longevidade
Um estudo recente examinou os perfis microbianos intestinais em coelhos e descobriu que certos micróbios, como Akkermansia e Christensenellaceae, estavam associados a uma vida reprodutiva mais longa e maior resiliência. Esses resultados sugerem que a seleção ou suplementação baseada no microbioma pode ser integrada a programas de melhoramento para aumentar a longevidade e a produtividade. Por aproveitamento de ferramentas como CAS Scientific Patent Explorer™, identificamos várias patentes demonstrando maior interesse comercial nessa área.
Por exemplo, uma patente recente descreve formulações probióticas estabilizadas para alimentos para pets que melhoram a palatabilidade e os desfechos de saúde. Outras patentes focam em probióticos multicepas e abordagens terapêuticas para corrigir a disbiose em gado e animais de companhia. Uma patente adicional Apresenta uma mistura de nutrientes microbianos desenvolvida para melhorar a saúde intestinal e o desempenho animal.
Novas classes de antibióticos
Descobertas recentes estão introduzindo novos agentes antimicrobianos com atividade de amplo espectro e toxicidade mínima, que visam preservar a microbiota intestinal benéfica enquanto combatem os patógenos. Um exemplo disso é Lariocidin, um peptídeo do tipo “laço” recentemente identificado, que demonstra alta eficácia em modelos animais e mostra potencial para superar mecanismos de resistência. Da mesma forma, descobriu-se que uma proteína bioativa das ostras de Sydney interrompe biofilmes bacterianos e aumenta a eficácia de antibióticos contra Staphylococcus aureus e Pseudomonas aeruginosa resistentes a medicamentos, sem toxicidade observada ou grandes perturbações no microbioma.
Terapia fotodinâmica antimicrobiana
A terapia fotodinâmica antimicrobiana (aPDT) é uma abordagem inovadora que utiliza um fotossensibilizador ativado pela luz, na presença de oxigênio, para produzir espécies reativas de oxigênio (ROS), que eliminam efetivamente os patógenos. Na medicina veterinária, a aPDT tem demonstrado potencial no tratamento da mastite em bovinos e ovinos, bem como de infecções cutâneas e otite em pequenos animais. Estudos demonstraram sua eficácia contra várias bactérias causadoras de mastite e cepas multirresistentes. No entanto, as limitações incluem a natureza tópica da aplicação do fotossensibilizador, devido à sensibilidade sistêmica à luz e à potencial toxicidade, o que exige mais investigações antes de um uso mais amplo na saúde animal.
Peptídeos antimicrobianos
Peptídeos antimicrobianos (AMPs) estão ganhando destaque como alternativas inovadoras aos antibióticos convencionais no cuidado com animais de criação e de estimação. Esses peptídeos, classificados em tipos sintetizados de forma não ribossomal e ribossomal, apresentam potente atividade bactericida contra diversos patógenos. Os AMPs não ribossomais, como polimixina e bacitracina, têm como alvo as membranas bacterianas e a síntese da parede celular, enquanto os AMPs sintetizados ribossomalmente, incluindo bacteriocinas como nisina e pediocina, interrompem vias biossintéticas e processos celulares essenciais. Sua biodegradabilidade, impacto ambiental mínimo e compatibilidade com outros tratamentos os tornam candidatos atrativos para aditivos alimentares e agentes terapêuticos. Apesar dessas vantagens, ainda persistem desafios como altos custos de produção, espectros antibacterianos restritos, potencial toxicidade e problemas de estabilidade durante a administração e o transporte. Os avanços na engenharia genética e de proteínas estão ajudando a superar essas limitações, abrindo caminho para que os AMPs desempenhem um papel mais relevante no manejo sustentável da saúde animal.
Estratégias imunomoduladoras
O fortalecimento do sistema imunológico natural dos animais por meio de estratégias imunomoduladoras oferece uma alternativa promissora às abordagens antimicrobianas tradicionais. Os principais métodos incluem o uso de probióticos, pré-bióticos, pós-bióticos e simbióticos, que dão apoio a um microbioma intestinal saudável e reforçam a resistência a patógenos nocivos. Os imunoestimulantes também são utilizados para ativar e fortalecer as respostas imunológicas, reduzindo assim a dependência de antibióticos. Além disso, programas de vacinação direcionados desempenham um papel crucial na prevenção de infecções causadas por patógenos resistentes, oferecendo proteção proativa e contribuindo para a saúde geral do rebanho e o manejo de doenças.
Fitoquímicos
Fitoquímicos são compostos bioativos derivados de plantas com benefícios comprovados para a saúde. Suas propriedades antimicrobianas, juntamente com a capacidade de aumentar a absorção de nutrientes, melhorar a saúde intestinal e reduzir a deterioração, os tornam alternativas naturais promissoras aos antibióticos na produção de ruminantes, suínos e aves.
Nanopartículas
Nanopartículas estão surgindo como alternativas promissoras aos antibióticos na saúde animal, devido a seus mecanismos antimicrobianos exclusivos e às suas propriedades físico-químicas. As nanopartículas de prata, cobre e compósitos têm demonstrado forte eficácia in vitro contra patógenos causadores de mastite, infecções uterinas e doenças nos cascos em bovinos leiteiros. A capacidade de desorganizar as membranas bacterianas, gerar espécies reativas de oxigênio e danificar estruturas intracelulares oferece uma abordagem inovadora para combater microrganismos resistentes a fármacos. Embora estudos iniciais sugiram segurança em baixas concentrações, preocupações sobre citotoxicidade e bioacumulação destacam a necessidade de mais pesquisas in vivo antes da aplicação veterinária ampla.
Terapia com ozônio
Terapia de ozônio vem ganhando atenção como uma alternativa segura e eficaz aos antibióticos na medicina veterinária. Suas fortes propriedades oxidantes permitem eliminar bactérias por meio da ruptura das membranas celulares e do DNA, além de oferecer efeitos imunomoduladores e antioxidantes em baixas doses. Utilizado em bovinos, ovinos, caprinos e equinos, o ozônio tem demonstrado sucesso no tratamento de mastite, metrite e distúrbios reprodutivos sem deixar resíduos no leite ou na carne, evitando assim períodos de carência.
Nutrigenética: alimentação por design
Tanto em humanos quanto em animais, a alimentação está intimamente ligada à saúde do microbioma intestinal. A nutrigenética, o estudo de como variações genéticas influenciam a resposta de um animal aos nutrientes, está transformando os serviços de saúde animal ao permitir uma nutrição de precisão adaptada aos perfis genéticos. Essencialmente, essa abordagem reconhece que variações genéticas individuais afetam a forma como os animais metabolizam os nutrientes e respondem às intervenções alimentares, com implicações diretas para a diversidade do microbioma e a prevalência de genes de resistência antimicrobiana.
No nível molecular, os nutrientes interagem com os genes por meio de vários mecanismos (veja a Figura 5). Compreender esses mecanismos permite o desenvolvimento de dietas direcionadas que sustentam a expressão gênica ideal, o desempenho fisiológico e uma composição benéfica do microbioma, ao mesmo tempo que reduz a pressão seletiva para a resistência antimicrobiana.
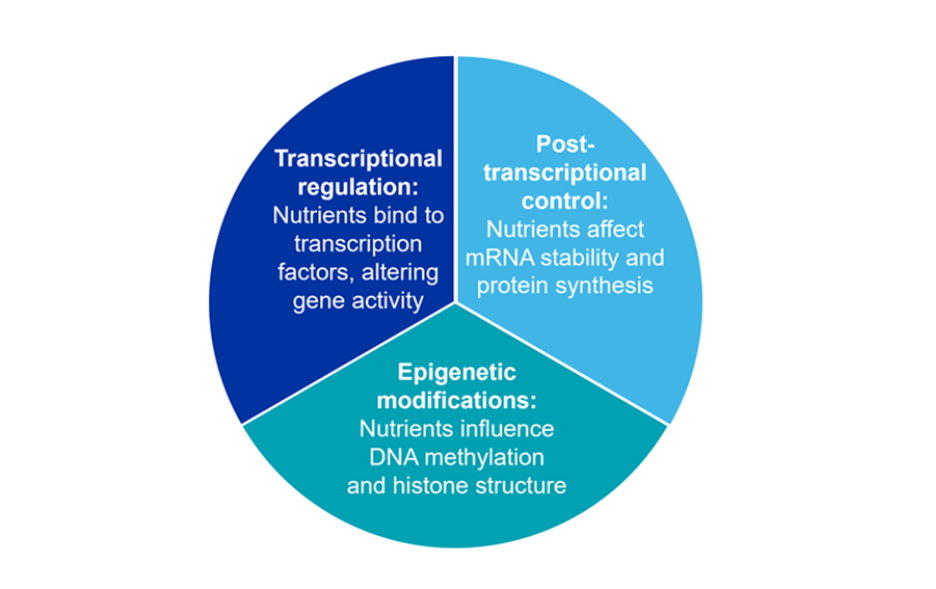
Figura 5: Mecanismos moleculares pelos quais os nutrientes interagem com os genes.
Aplicações e benefícios para a saúde
- Produção animal: A nutrigenética encontrou amplas aplicações na produção animal, oferecendo estratégias nutricionais direcionadas que se alinham aos perfis genéticos para melhorar a saúde e a produtividade. Pesquisas revelaram que a genética do hospedeiro pode influenciar a composição microbiana intestinal, com variações genéticas específicas afetando a eficiência alimentar por meio de vias mediadas pelo microbioma. Na avicultura, o perfil genético permitiu a formulação de rações que aumentam as taxas de crescimento, a eficiência da conversão alimentar e a resiliência imunológica, ao mesmo tempo que promovem comunidades microbianas benéficas que resistem à colonização por patógenos. Na suinocultura, as abordagens da nutrigenética têm ajudado a identificar suínos com características superiores de aproveitamento de nutrientes, permitindo uma alimentação de precisão que favorece o desempenho reprodutivo e reduz desperdícios. Da mesma forma, em bovinos leiteiros, dietas baseadas no genótipo demonstraram melhorar a produção de leite e reduzir a incidência de distúrbios metabólicos, como cetose e acidose. Indo além da produção animal tradicional, ovinos e caprinos estão sendo estudados quanto a características nutrigenéticas que influenciam a eficiência reprodutiva, a função imunológica e a adaptação a ambientes com escassez de nutrientes.
- Equinos: A nutrigenética está sendo explorada em cavalos para o manejo de síndromes metabólicas, o suporte ao desempenho de equinos atletas e a prevenção de condições como a laminite por meio de estratégias nutricionais personalizadas. Essas aplicações são relevantes em contextos competitivos e terapêuticos, nos quais a saúde e a recuperação ideais são essenciais.
- Animais de companhia: a nutrigenética está ganhando espaço no manejo de condições de saúde específicas de raças em cães e gatos, como obesidade, alergias alimentares e distúrbios metabólicos. Dietas personalizadas com base em predisposições genéticas estão sendo desenvolvidas para melhorar a longevidade e o bem-estar geral. Essas abordagens também favorecem a saúde imunológica e digestiva, contribuindo para uma melhor qualidade de vida e para a redução de intervenções veterinárias.
Olhando para o futuro, a integração bem-sucedida da nutrigenética na nutrição animal dependerá de diversas prioridades estratégicas: a simplificação dos processos de triagem genética, o desenvolvimento de modelos de alimentação escaláveis e adaptáveis que considerem as interações do microbioma, a ampliação das pesquisas para incluir espécies sub-representadas e o estabelecimento de marcos regulatórios claros. Ao adotar esses avanços, produtores, veterinários e tutores de animais podem migrar de abordagens alimentares genéricas para estratégias de nutrição de precisão que se alinham ao potencial genético e às necessidades de saúde de cada animal.
A saúde intestinal significa bem-estar geral do animal
O avanço da saúde animal exige uma abordagem holística que integre a ciência do microbioma intestinal, os conhecimentos da nutrigenética e alternativas inovadoras à resistência antimicrobiana. Juntos, esses campos oferecem ferramentas poderosas para fortalecer a imunidade, otimizar a nutrição e reduzir a dependência de antibióticos. À medida que continuamos a compreender a importância do microbioma intestinal, novos achados serão aplicados não apenas à saúde humana, mas também às fontes de alimentos e aos animais de companhia que interagem conosco e com o nosso ambiente.