의사가 암을 발견하는 순간 치료를 시작할 수 있다면 어떨까요? 최근 테라노스틱스('치료'와 '진단'의 합성어)의 발전으로 이 '직접 보고 치료하는' 접근 방식이 임상에서 더 많이 사용되고 있습니다. 복잡한 질병에 대한 고도의 표적 치료를 가능하게 함으로써 의료 서비스를 혁신하고 있는 핵의학에 의존하고 있습니다.
핵의학은 전통적으로 진단 분야로 알려져 왔지만, 이제는 동일한 분자 물질을 영상 진단과 치료에 사용하는 혁신을 통해 치료 분야로도 발전하고 있습니다. 이러한 변화는 임상의가 암, 심혈관 및 신경 질환에 접근하는 방식을 재정의하여 보다 개인화되고 효과적인 치료 전략을 제공하고 있습니다.
진단과 치료 기능을 결합한 치료제를 개발하려면 표적 메커니즘, 이미징 특성, 치료 효능에 대한 통합적인 분석이 필요합니다. 치료 플랫폼에서 작업하는 신약 개발 팀은 CAS BioFinder를 사용하여 상호 연결된 생물학 및 화학 데이터에 액세스하여 표적-리간드 상호작용, 생물 분포 프로필, 진단-치료 결합 접근법에 대한 안전성 고려 사항을 평가할 수 있습니다.
자세히 알아보세요.핵의학의 기본 구성 요소
핵의학 치료의 핵심은 방사성 동위원소(방사성 핵종)와 운반체 분자를 결합하여 영상 또는 치료를 위해 표적 방사선을 전달하는 화합물인 방사성 의약품입니다. 이러한 약제는 체내에서 안전하고 효과적으로 운반될 수 있도록 킬레이터에 의해 안정화되는 경우가 많습니다(그림 1 참조).
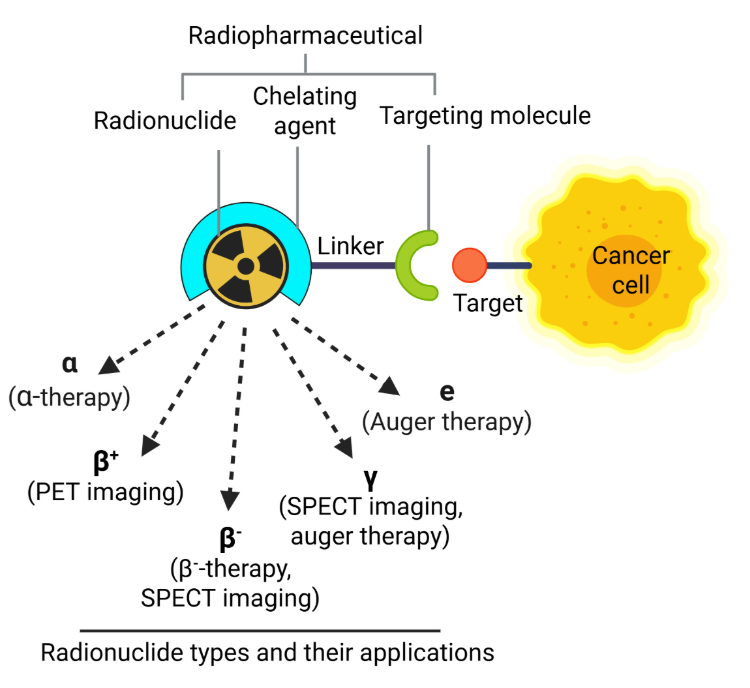
이러한 방사성 물질은 방출 특성에 따라 PET 스캔(양전자 방출 단층 촬영)과 SPECT 스캔(단일광자 방출 컴퓨터 단층 촬영)에서 진단적으로 사용하거나 DNA 손상을 유도하여 병든 세포를 파괴하는 치료적으로 사용할 수 있습니다. 이러한 손상에는 단일 가닥 단선(SSB)과 이중 가닥 단선(DSB)이 포함되며, 방출되는 방사선의 유형에 따라 그 정도와 성격이 결정됩니다.
알파 입자 방출체(예: 라듐-223, 악티늄-225, 아스타틴-211)는 선형 에너지 전달(LET)이 높고 경로 길이가 짧아 세포사멸 및 발열 경로를 활성화하는 조밀하고 국소화된 DSB를 유발합니다. 오거 전자 방출체(예: 요오드-125, 인듐-111)는 또한 짧은 범위와 높은 LET로 인해 고도로 국소화된 DSB를 생성합니다.
반면 베타 입자 방출체(예: 루테튬-177, 이트륨-90)는 LET가 낮고 주로 활성산소종(ROS)의 생성을 통해 간접적인 DNA 손상을 유도하여 SSB 또는 DSB와 산화 스트레스를 유발합니다. 이러한 병변은 염기 절제 복구(BER), 뉴클레오티드 절제 복구(NER), 비동일 종말 결합(NHEJ), 상동 재조합(HR) 등의 DNA 복구 메커니즘을 활성화합니다.
그러나 암세포는 종종 DNA 복구 능력이 손상되어 방사성 의약품으로 인한 세포 독성에 취약한 경우가 많습니다. 치료 효과를 높이기 위해 방사성 의약품과 폴리(ADP-리보스) 중합효소(PARP) 억제제와 같은 DNA 복구 억제제를 병용하는 전략이 활발히 연구되고 있습니다.
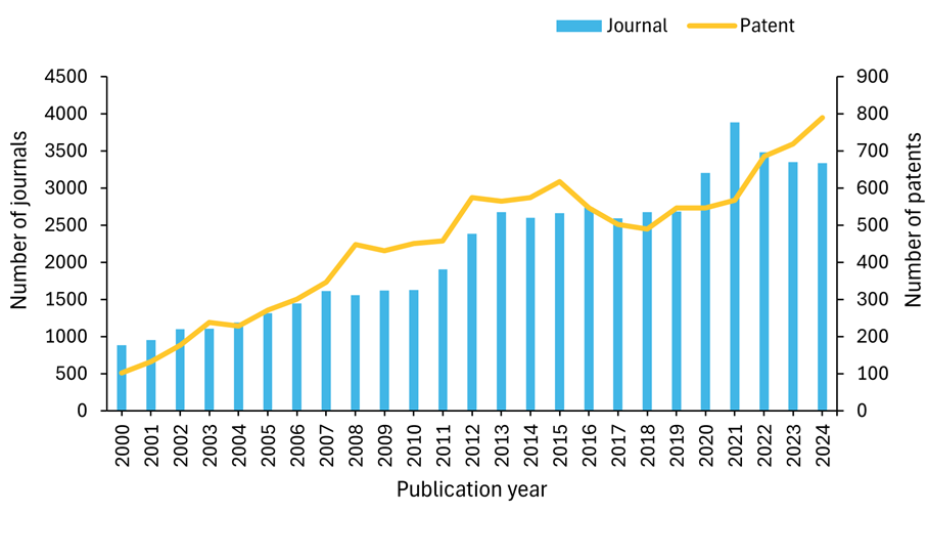
핵의학 분야의 연구 환경을 더 잘 이해하기 위해 사람이 엄선한 최대 규모의 과학 정보 저장소인 CAS Content CollectionTM을 조사한 결과, 지난 20년 동안 출판물의 양이 기하급수적으로 증가한 것을 발견했습니다. 특히 2020년 이후 특허 활동이 크게 증가하여 2024년에는 800건에 육박하는 특허가 출원되었으며, 이는 핵의학 기술에 대한 상업적 관심과 혁신이 증가하고 있음을 시사합니다(그림 2 참조).
현재 FDA 승인을 받은 방사성의약품은 54개 진단제와 13개 치료제로 약 67개에 달합니다. 현재 승인된 모든 치료용 방사성 의약품은 암 치료를 목적으로 합니다(그림 3 참조).
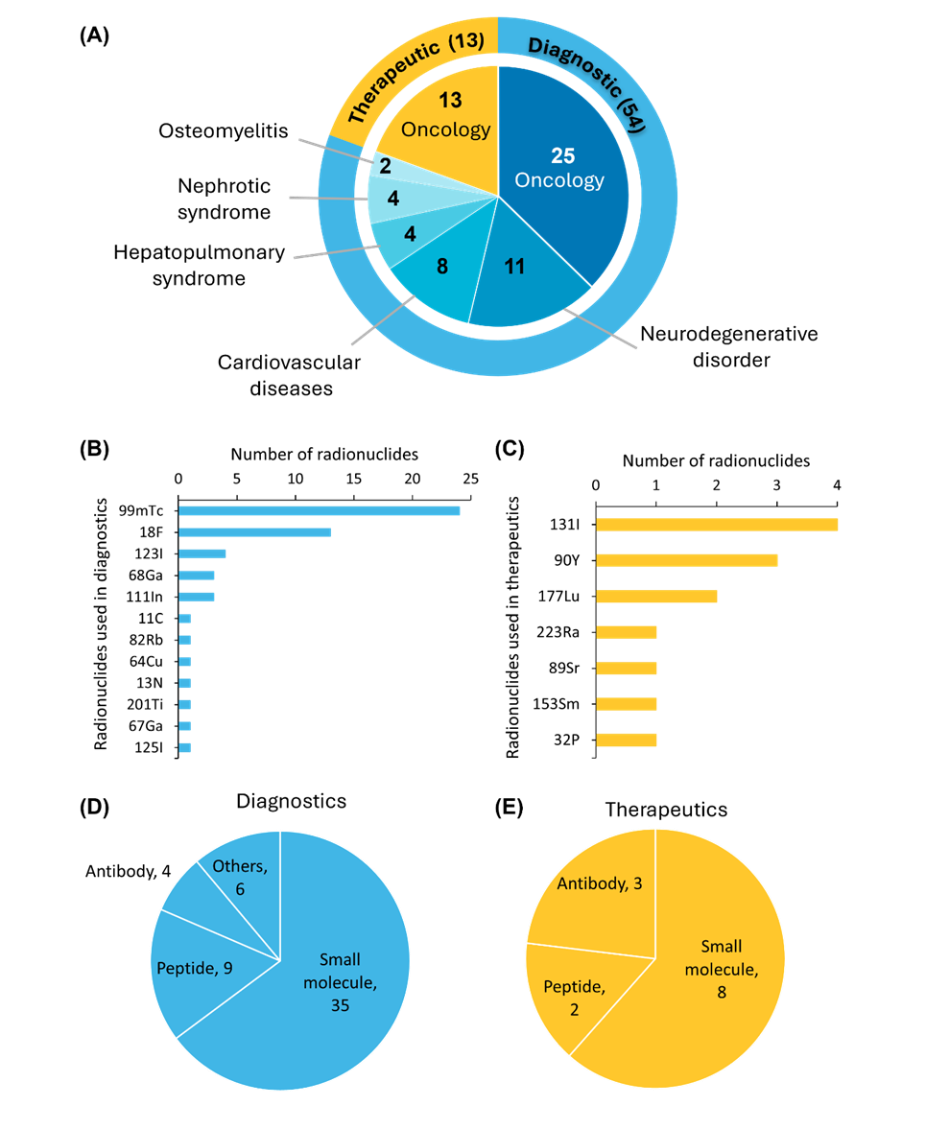
FDA 승인 방사성 의약품 중 테크네튬-99m(⁹⁹ᵐTc)은 진단 영상에 가장 많이 사용되는 방사성 핵종이며, 요오드-131(¹³¹I)은 여전히 치료 용도로 가장 많이 사용되고 있습니다. 표적 벡터의 경우, 저분자는 약동학적으로 유리하고 개발이 용이하기 때문에 가장 많이 사용됩니다.
신경내분비 종양의 진단 및 치료를 위한[68Ga]/[177Lu]Ga-DOTA-TATE의 FDA 승인에 이어 펩타이드 기반 제제도 임상적으로 중요한 의미를 갖게 되었습니다. 항체 기반 방사성의약품은 생체 내 결합 친화력이 높아 이미징 정밀도와 치료 효능을 향상시키는 중요한 역할을 합니다. 또한 단백질과 혈청 알부민을 기반으로 하는 방사성 의약품도 승인된 소규모 약제 그룹에 포함됩니다.
핵의학 분야의 최신 돌파구
방사성 의약품, 영상 촬영 방식, 분자 표적화, 동위원소 생산 분야의 획기적인 혁신이 핵의학 발전을 주도해 왔습니다. 예를 들어 2020년부터 2024년 사이의 짧은 기간 동안 임상 시험용 방사성의약품의 수는 약 40개에서 170개로 크게 증가했습니다. 이러한 빠른 성장세는 신경내분비 종양 치료를 위한 Lutathera®([177Lu]Lu-DOTA-TATE)와 PSMA 양성 전이성 전립선암 치료를 위한 Pluvicto®([177Lu]Lu-PSMA-617) 등 선구적인 약제의 규제 승인과 상업적 성공에 힘입어 표적 방사선 리간드 치료의 새로운 기준을 세운 데 따른 것으로 보입니다.
주목할 만한 분야에는 다음이 포함됩니다.
- 테라노스틱스: 이 역동적인 분야에서 최근 몇 년간 가장 중요한 혁신 중 하나는 진단 및 치료에 사용되는 유사한 방사성 의약품 화합물인 치료용 방사성 의약품의 개발입니다. 예를 들어, 최근 FDA 승인을 받은 갈륨-68(Locametz)로 표지된 전립선 특이적 막 항원(PSMA) 리간드는 PET 영상을 통해 전립선암을 발견하는 데 사용할 수 있습니다. 동일한 PSMA 리간드를 루테튬-177(Pluvicto)로 표지하면 암 치료를 위한 표적 방사선 치료를 제공할 수 있습니다. 이 접근 방식은 정밀 의학의 정점에 해당하는 것으로, 임상의가 매우 정확하게 질병 부위를 식별한 다음 건강한 조직을 보존하면서 동일한 표적에 직접 방사선 치료를 전달할 수 있습니다.
- 표적 알파 치료: 양성자 2개와 중성자 2개로 구성된 헬륨 핵인 알파 입자는 높은 LET와 제한된 조직 침투력(50-100μm)을 나타내므로 주변의 건강한 조직 손상을 최소화하면서 개별 암세포에 치명적인 방사선량을 전달하는 데 이상적입니다. 이 속성은 정밀 종양학의 획기적인 발전인 표적 알파 치료(TAT)의 효과를 뒷받침합니다.
처음에는 ²²³Ra-염화물(Xofigo®)로 예시된 TAT는 ²²⁵Ac-PSMA-617과 같은 약제로 확장되어 전이성 거세 저항성 전립선암(mCRPC)에서 91%의 환자가 전립선 특이 항원(PSA) 수치 감소를 경험하고 15개월의 중앙 생존율을 보이는 등 놀라운 임상 결과를 보여 주었습니다. 동위원소 생산의 발전은 이제 대규모 제조와 ²¹²Pb와 같은 새로운 알파 이미터 개발을 지원합니다. 임상 시험에서는 위장 췌장 신경내분비 종양에 대한 RayzeBio의 3상 RYZ101 시험을 포함하여 다양한 암 유형에 걸쳐 다양한 알파 방출 치료법을 연구하고 있습니다.
기술 혁신은 TAT의 잠재력을 더욱 향상시키고 있습니다. 여기에는 이중 특이적 항체, 개선된 접합 화학, 면역 요법 및 DNA 손상 반응 억제제와의 병용 전략이 포함됩니다. 이러한 발전을 통해 TAT는 기존의 베타 방출 방사선 치료법에 대한 내성을 극복할 수 있는 혁신적인 접근법으로 자리매김했습니다.
- 차세대 운반체: 핵의학에서 차세대 운반체 분자는 표적 특이성, 약물동력학 및 다중 모드 기능을 향상시키는 첨단 전달 플랫폼을 의미합니다. 최근 연구는 기존 운반체의 유망한 대안으로 바이클릭 펩타이드에 초점을 맞추고 있는데, 이는 항체 유사 친화도(나노몰 범위)가 뛰어나고 조직 침투력이 우수하며 신장 청소율이 빠르며, 주사 후 5분 만에 높은 종양 흡수율(19.5 ± 3.5 %ID/g)과 우수한 영상 대비를 보인 EphA2 표적 BCY18469를 예로 들 수 있습니다.
초소형 나노 입자(<10nm)는 다양한 악성 종양의 이중 PET/광학 이미징을 위한 임상 시험에서 코넬 도트를 사용하여 임상 전환에 성공했습니다. 이 분야는 열악한 방사성 표지 조건(최대 95°C, pH 3.6-11.0)에서 안정성이 향상된 DARPin(설계된 안키린 반복 단백질), 어피바디 및 나노바디를 포함한 대체 스캐폴드 단백질로 발전하고 있습니다. 높은 표적 친화성을 유지하면서 특정 세포 구획에 스마트하고 속도감 있게 전달할 수 있는 모듈형 나노 수송체를 함께 사용합니다.
이러한 혁신은 표적 외 독성을 줄이고, 최적의 종양 축적을 위한 순환 시간을 연장하며, 능동-수동 표적화 전략의 조합을 가능하게 함으로써 핵의학에서 중요한 과제를 해결합니다. 또한 단일 플랫폼 내에서 진단 영상과 치료적 개입을 제공할 수 있는 치료제의 개발을 지원하여 차세대 캐리어를 개인 맞춤형 정밀 의료를 위한 혁신적인 도구로 자리매김하고 있습니다.
- 초기 단계 질환에 대한 초기 치료: 핵의학은 최근 몇 년 동안 임상적으로 크게 성장했지만, 전통적으로 진행성 암 환자를 위한 최후의 완화 치료로 여겨져 왔습니다. 이러한 패러다임은 이제 활발한 임상 연구를 통해 극적으로 변화하고 있습니다. 예를 들어, 소마토스타틴 수용체 2(SSTR2)와 PSMA 표적 방사성 의약품 치료는 초기 단계의 질병에서 가능성을 보이고 있습니다. 특히, [¹⁷⁷Lu]Lu-DOTA-TATE는 진행성 위장관 췌장 신경내분비 종양의 초기 치료제로 사용했을 때 무진행 생존기간을 통계적으로 유의미하고 임상적으로 의미 있게 개선한 것으로 나타났습니다.
동시에 ¹⁷⁷Lu 표지 PSMA 표적 화합물은 치료 경험이 없는 전이성 거세 저항성 질환, 전이성 호르몬 민감성 암, 과형 전이성 또는 생화학적으로 재발한 질환, 국소 진행성 또는 고위험군 등 다양한 전립선암 시나리오에 대해 광범위한 임상 시험을 진행 중입니다. 말기 완화 치료에서 초기 치료 개입으로 확대되는 이러한 변화는 방사성 의약품이 포괄적인 암 치료 전략에 통합되는 방식에 근본적인 변화를 의미하며, 질병이 아직 치료 가능한 초기 단계에 있는 환자에게 더 효과적인 결과를 제공할 수 있을 것으로 기대됩니다.
- 개인 맞춤형 방사성 의약품 치료에서의 AI: AI 모델은 이미지 재구성 및 개선, 자동화된 병변 감지, 장기/종양 식별을 용이하게 하여 개인화된 선량 측정 계산을 가능하게 합니다. 이 통합은 방사성 의약품 치료의 일상적이고 신뢰할 수 있는 개인 맞춤화를 실현하여 치료 효과와 환자 결과를 개선하는 것을 목표로 합니다.
이러한 혁신 기술들이 규제 절차를 거쳐 임상 적용에 점점 더 가까워지고 있음을 알 수 있습니다. 그림 4는 임상 시험용 의약품을 1상, 2상, 3상으로 분류하고 각 세그먼트의 용도에 따라 진단용, 치료용, 치료제로 구분하여 색상으로 표시합니다. 데이터에 따르면 항암제는 모든 단계, 특히 32개의 약물이 포함된 임상 1상에서 가장 많이 사용되는 것으로 나타났습니다. 이러한 추세는 종양학에서 표적 방사성 핵종 치료 및 테라노스틱에 대한 강조가 증가하고 있음을 반영하며, 방사성 의약품을 이용한 개인 맞춤형 치료 전략으로의 전환을 강조하는 최근의 문헌과도 일치합니다. 비록 그 정도는 적지만 다른 질병 분야에서도 나타나고 있어 암을 넘어 응용 분야가 확대되고 있음을 알 수 있습니다.
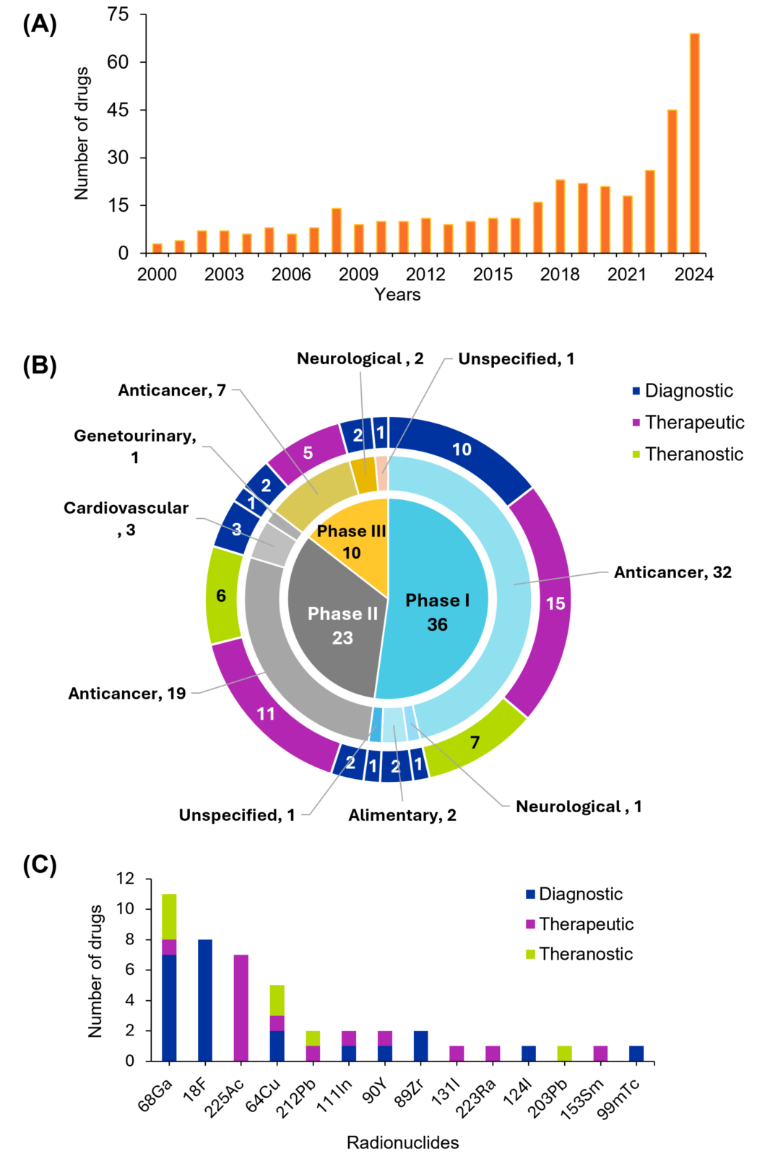
임상 시험 분석에 따르면 갈륨-68(⁶⁸Ga)과 플루오린-18(¹⁸F)은 반감기와 양전자 방출 특성으로 인해 PET 영상에서 널리 사용되고 있으며, 진단 애플리케이션에서 두드러진 활약을 보이고 있습니다. 치료 분야에서는 악티늄-225(²²⁵Ac) 납이 표적 방사선 치료에 이상적인 알파 방출 특성으로 알려져 있습니다. 구리-64(⁶⁴Cu) 및 납-212(²¹²Pb) 같은 방사성 핵종은 영상과 치료를 통합하는 치료 패러다임의 성장을 지원하는 이중 사용 가능성을 보여줍니다.
핵의학을 개념에서 임상으로 옮기다
핵의학을 임상 진료에 도입하는 데는 여러 가지 어려움이 있는데, 특히 높은 비용과 의료용 동위원소의 제한된 가용성 문제가 있습니다. 또한 훈련된 핵의학 전문가와 방사성 약사가 부족하고 인프라의 한계로 인해 첨단 영상 및 치료에 대한 접근성이 제한되고 있습니다. 이 현상은 저소득 국가에서 가장 두드러집니다.
동위원소 비축량 확대, 인력 확충, 치료 접근성 향상은 모두 장기적으로 해결해야 할 문제이며, 이를 해결하기 위해서는 전담 자원이 필요합니다. 그러나 이 분야의 투자 수준과 예상 성장률을 고려할 때 미래는 유망할 수 있습니다.
2024년 핵의학 부문은 설비 투자 급증과 전략적 통합에 힘입어 괄목할 만한 성장을 이루었습니다. PitchBook에 따르면 이 분야에 대한 총 투자액은 148억 6,000만 달러로 급증하여 2023년에 투자된 금액의 세 배가 넘었습니다. 이러한 흐름은 주요 제약 회사들이 확장되는 시장에서 입지를 강화하기 위해 신속하게 움직이면서 이루어진 일련의 대규모 인수합병으로 더욱 증폭되었는데, 대표적인 예로 AstraZeneca가 표적 알파 치료법 개발을 위해 Fusion Pharmaceuticals를 24억 달러에 인수한 사례가 있습니다.
테라노스틱과 핵의학의 광범위한 적용에는 여전히 장애물이 남아 있지만, 더 많은 혁신이 암과 기타 쇠약해지는 질병에 대한 "직접 보고 치료하는 방식"에 더욱 가까워지고 있습니다.
관련 사례 연구: 합리적인 가격의 고품질 의약품을 보호하기 위한 새로운 역량 구축 - Sandoz가 CAS와 협력하여 전 세계 환자들에게 합리적인 가격의 고품질 의약품을 제공하기 위해 맞춤형 R&D 역량을 구축한 방법을 확인하세요.
사례 연구를 읽어보세요.