Gain new perspectives for faster progress directly to your inbox.

To achieve decarbonization, we must develop reliable, clean energy sources at a massive scale. One key to this transition from fossil fuels will be hydrogen, which can be stored for long periods of time and then used as a natural gas replacement to power turbines or in clean-burning fuel cells. The International Energy Agency (IEA) notes that to reach net-zero emissions by 2050, the world needs hydrogen to satisfy at least 10% of total energy consumption.
Hydrogen production is currently taking place, but 95% of U.S. hydrogen production is via burning fossil fuels to isolate it — an emission-intensive process that does not contribute to decarbonization. This is why clean hydrogen production from splitting water molecules with renewable energy sources is important. Using solar energy to split water into its hydrogen and oxygen components has even been called one of the “holy grails” of chemistry.
However, achieving this reaction at a large enough scale for commercialization remains a challenge. It requires very specific properties to be present in the materials involved, and scientists have been working for decades to find suitable candidates. My colleagues and I at CAS recently explored the state of research on photocatalysis, and our findings from the CAS Content CollectionTM show that the scientific community is making significant strides toward commercial viability.
Important work remains to be done, but it’s an exciting time to be involved in this research.
What is photocatalysis?
Photocatalysis is a chemical reaction performed by irradiating semiconducting materials usually dispersed in an aqueous medium, forming of electron and hole pairs. First demonstrated in 1972 by Kenichi Honda and Akira Fujishima, this reaction occurred when light shone onto a titanium dioxide electrode submerged in water. The holes created by the reaction can draw more electrons from the surrounding water molecules, thereby splitting water into oxygen and hydrogen.
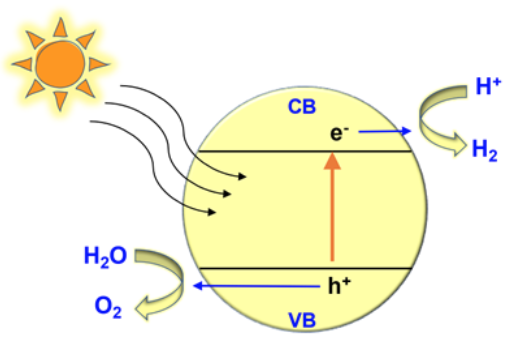
This process results in emission-free hydrogen production, which can then be stored for use as a clean-burning fuel and fossil fuel replacement. Photocatalysis is also promising for other types of environmental remediation, particularly water purification and destroying wastewater contaminants. It has successfully degraded microorganisms and non-organic substances like dyes and chemicals in water. This process may be feasible for remediating pesticides in soil — a key advancement in the technology beyond reactions taking place in water or a solution.
Producing clean hydrogen without complex infrastructure is arguably the most urgent application of this process. Photocatalysis can be conducted with a powder added to water, which is much simpler than the other emission-free way to produce hydrogen via solar-powered electrolyzers. As a result, researchers have accelerated their efforts to find the right photocatalysts and make this process commercially viable.
The latest research on photocatalysis
By analyzing the CAS Content CollectionTM, we found that the most common concepts cited in journal and patent literature addressed photocatalytic reactions and the material properties of the photocatalysts themselves. This makes sense because photocatalysts must use visible light to be economically competitive with fossil fuels. Most of the sunlight falls in the visible range, so we need to use it as an affordable way to drive large-scale reactions. Specifically, solar-to-hydrogen (STH) efficiency must be at least 6-10% or higher.
What properties do photocatalysts need to achieve this? First, their conduction and valence bands must be in certain positions (Fig. 2). The placement of these bands allows for the movement of electrons — a property of semiconductors and a necessary part of the photocatalytic reaction. The conduction band must be negative at zero volts, and the valence band should be positive at 1.23 volts or more. If a photocatalyst’s bands are not in these positions, there won’t be enough energy for the reaction.
Second, the band gap, i.e., the space between the conduction and valence bands, must be able to absorb visible light. To meet this criteria, the band gap should be between 1.23 and 3.1 volts. Titanium dioxide is a stable material with bands in suitable positions, but it can’t absorb visible light, so it won’t be economical for large-scale photocatalysis. Researchers in the last 10 years have turned to other photocatalysts instead.
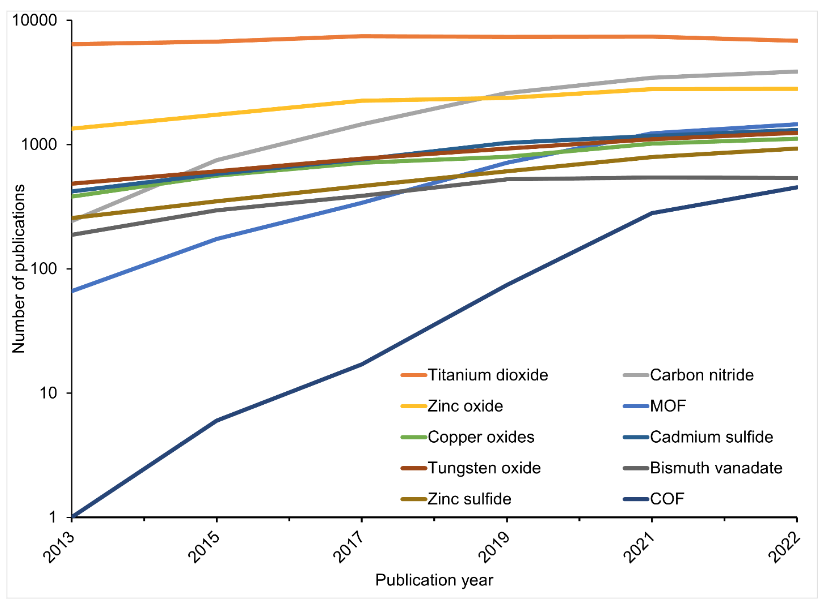
As you can see in Figure 3, the most-studied photocatalysts other than titanium dioxide include zinc oxide, carbon nitride, metal organic frameworks (MOF), and more:
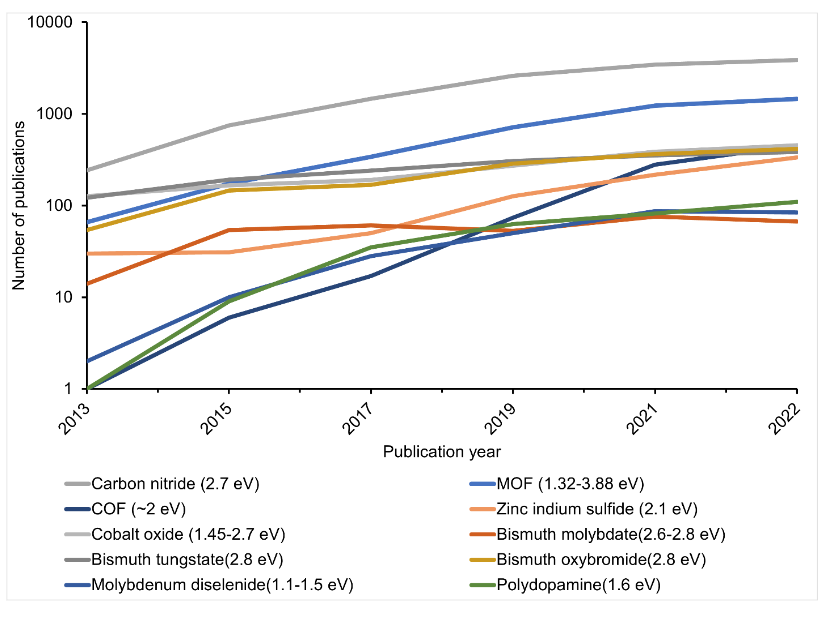
The common property among these photocatalysts is their band gap, which is below 3.0 volts and therefore capable of absorbing visible light (Fig. 4). Despite having these properties, however, the photocatalysts being examined still have shortcomings. As a result, scientists are exploring more options to improve reaction efficiency.
Challenges ahead
As our analysis shows, with the exception of using concentrated sunlight, the photocatalysts under consideration haven’t reached the STH threshold of 6% (Fig. 4).
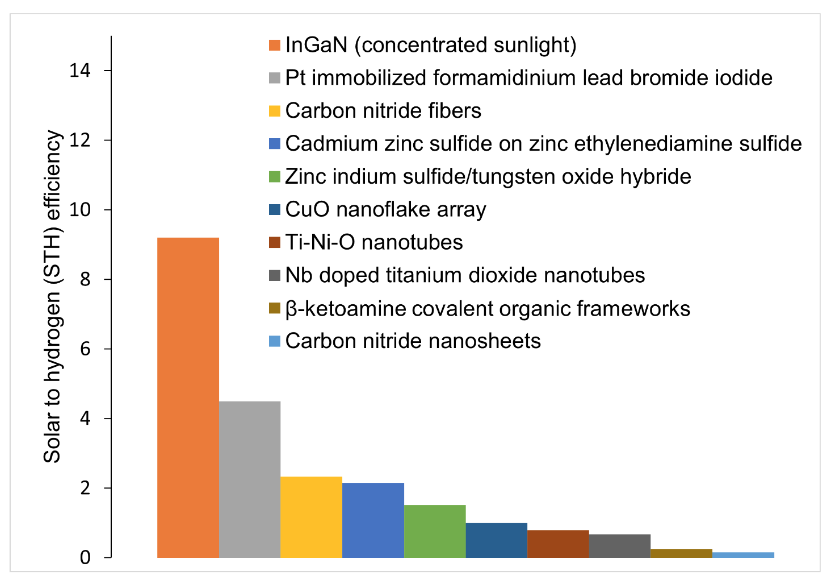
Researchers are trying to combine catalysts to get the right properties of band positions and band gaps. The most promising technique under consideration is called step-scheme (S-scheme) heterojunction or combining two photocatalysts with a staggered band structure. The position of these bands when working together can drive electrons to carry out the photocatalytic reaction robustly and efficiently — a major step towards expanding the use of this technology.i
Yet, it’s never as simple as just combining two catalysts. Once you combine materials, there can be other unexpected results. For example, electrons and holes can recombine before they carry out the desired reaction. Researchers are exploring co-catalysts to control this process of recombination. Co-catalysts can be deposited on the surface of photocatalysts, which speeds up the utilization of the generated charge carriers. Several co-catalyst materials are being researched, showing that the future of photocatalysis is bright even if there is more work to do.
Making a hydrogen-fueled future a reality
Splitting water molecules into hydrogen and oxygen without producing emissions is no simple task — scientists have been working on this for decades. There is still considerable progress to make before this process is cost-effective enough to replace fossil fuels with clean hydrogen.
While the challenges are formidable, there is no reason to believe they’re insurmountable. A recent analysis of the CAS Content CollectionTM proves that innovative research on photocatalysts is growing. As techniques such as S-scheme configuration and co-catalyst use increase, so will breakthroughs in STH efficiency and the ability to commercialize this technology.
With continued experimentation and improvements in material science, the world can achieve the promise of large-scale hydrogen production and make a decarbonized future into a reality.



